Universal CAR 2.0 to overcome current limitations in CAR therapy
- PMID: 38962014
- PMCID: PMC11219820
- DOI: 10.3389/fimmu.2024.1383894
Universal CAR 2.0 to overcome current limitations in CAR therapy
Abstract
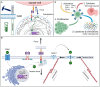
Chimeric antigen receptor (CAR) T cell therapy has effectively complemented the treatment of advanced relapsed and refractory hematological cancers. The remarkable achievements of CD19- and BCMA-CAR T therapies have raised high expectations within the fields of hematology and oncology. These groundbreaking successes are propelling a collective aspiration to extend the reach of CAR therapies beyond B-lineage malignancies. Advanced CAR technologies have created a momentum to surmount the limitations of conventional CAR concepts. Most importantly, innovations that enable combinatorial targeting to address target antigen heterogeneity, using versatile adapter CAR concepts in conjunction with recent transformative next-generation CAR design, offer the promise to overcome both the bottleneck associated with CAR manufacturing and patient-individualized treatment regimens. In this comprehensive review, we delineate the fundamental prerequisites, navigate through pivotal challenges, and elucidate strategic approaches, all aimed at paving the way for the future establishment of multitargeted immunotherapies using universal CAR technologies.
Keywords: CAR (chimeric antigen receptor) T-cell therapy; antibody therapies; cancer immune cell therapy; iPSC (induced pluripotent stem cell); individualized cancer therapy.
Copyright © 2024 Schlegel, Werbrouck, Boettcher and Schlegel.
Conflict of interest statement
PS and CW are inventors of patents in the field of indirect CAR technologies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

