Immunogenicity of PvCyRPA, PvCelTOS and Pvs25 chimeric recombinant protein of Plasmodium vivax in murine model
- PMID: 38962015
- PMCID: PMC11219565
- DOI: 10.3389/fimmu.2024.1392043
Immunogenicity of PvCyRPA, PvCelTOS and Pvs25 chimeric recombinant protein of Plasmodium vivax in murine model
Abstract
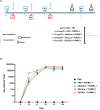
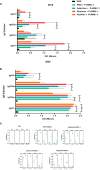
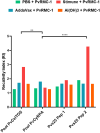
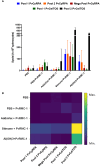
In the Americas, P. vivax is the predominant causative species of malaria, a debilitating and economically significant disease. Due to the complexity of the malaria parasite life cycle, a vaccine formulation with multiple antigens expressed in various parasite stages may represent an effective approach. Based on this, we previously designed and constructed a chimeric recombinant protein, PvRMC-1, composed by PvCyRPA, PvCelTOS, and Pvs25 epitopes. This chimeric protein was strongly recognized by naturally acquired antibodies from exposed population in the Brazilian Amazon. However, there was no investigation about the induced immune response of PvRMC-1. Therefore, in this work, we evaluated the immunogenicity of this chimeric antigen formulated in three distinct adjuvants: Stimune, AddaVax or Aluminum hydroxide (Al(OH)3) in BALB/c mice. Our results suggested that the chimeric protein PvRMC-1 were capable to generate humoral and cellular responses across all three formulations. Antibodies recognized full-length PvRMC-1 and linear B-cell epitopes from PvCyRPA, PvCelTOS, and Pvs25 individually. Moreover, mice's splenocytes were activated, producing IFN-γ in response to PvCelTOS and PvCyRPA peptide epitopes, affirming T-cell epitopes in the antigen. While aluminum hydroxide showed notable cellular response, Stimune and Addavax induced a more comprehensive immune response, encompassing both cellular and humoral components. Thus, our findings indicate that PvRMC-1 would be a promising multistage vaccine candidate that could advance to further preclinical studies.
Keywords: BALB/c; immunization; immunogenicity; in silico simulation; multistage chimeric protein; plasmodium vivax; vaccine.
Copyright © 2024 Matos, Soares, Rodrigues-da-Silva, Rodolphi, Albrecht, Donassolo, Lopez-Camacho, Ano Bom, Neves, Conte, Pratt-Riccio, Daniel-Ribeiro, Totino and Lima-Junior.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Characterization of T and B cell epitopes in PvCyRPA by studying the naturally acquired immune response in Brazilian Amazon communities.Sci Rep. 2024 Nov 9;14(1):27343. doi: 10.1038/s41598-024-72671-x. Sci Rep. 2024. PMID: 39521783 Free PMC article.
-
Induction of Multifunctional Broadly Reactive T Cell Responses by a Plasmodium vivax Circumsporozoite Protein Recombinant Chimera.Infect Immun. 2015 Sep;83(9):3749-61. doi: 10.1128/IAI.00480-15. Epub 2015 Jul 13. Infect Immun. 2015. PMID: 26169267 Free PMC article.
-
Construction, Expression, and Evaluation of the Naturally Acquired Humoral Immune Response against Plasmodium vivax RMC-1, a Multistage Chimeric Protein.Int J Mol Sci. 2023 Jul 18;24(14):11571. doi: 10.3390/ijms241411571. Int J Mol Sci. 2023. PMID: 37511330 Free PMC article.
-
Evaluation of Plasmodium vivax Cell-Traversal Protein for Ookinetes and Sporozoites as a Preerythrocytic P. vivax Vaccine.Clin Vaccine Immunol. 2017 Apr 5;24(4):e00501-16. doi: 10.1128/CVI.00501-16. Print 2017 Apr. Clin Vaccine Immunol. 2017. PMID: 28179403 Free PMC article.
-
Finding the sweet spots of inhibition: understanding the targets of a functional antibody against Plasmodium vivax Duffy binding protein.Int J Parasitol. 2012 Nov;42(12):1055-62. doi: 10.1016/j.ijpara.2012.09.006. Epub 2012 Oct 12. Int J Parasitol. 2012. PMID: 23068913 Free PMC article. Review.
Cited by
-
Anti-tumor effect and immune-related mechanism study of compound aluminum sulfate injection in transplanted tumor-bearing mice.Front Immunol. 2025 May 1;16:1583275. doi: 10.3389/fimmu.2025.1583275. eCollection 2025. Front Immunol. 2025. PMID: 40375992 Free PMC article.
-
Characterization of T and B cell epitopes in PvCyRPA by studying the naturally acquired immune response in Brazilian Amazon communities.Sci Rep. 2024 Nov 9;14(1):27343. doi: 10.1038/s41598-024-72671-x. Sci Rep. 2024. PMID: 39521783 Free PMC article.
References
-
- World Health Organization . World malaria report. Geneva: (2023).
-
- World Health Organization . World malaria report. Geneva: (2022).
-
- Saúde Md . Sistema de informação da vigilância epidemiológica/sivep - malária 2022 (2022). Available online at: http://200.214.130.44/sivep_malaria/.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

