Modulatory effects of cancer stem cell-derived extracellular vesicles on the tumor immune microenvironment
- PMID: 38962016
- PMCID: PMC11219812
- DOI: 10.3389/fimmu.2024.1362120
Modulatory effects of cancer stem cell-derived extracellular vesicles on the tumor immune microenvironment
Abstract
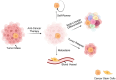
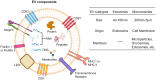
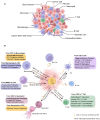
Cancer stem cells (CSCs), accounting for only a minor cell proportion (< 1%) within tumors, have profound implications in tumor initiation, metastasis, recurrence, and treatment resistance due to their inherent ability of self-renewal, multi-lineage differentiation, and tumor-initiating potential. In recent years, accumulating studies indicate that CSCs and tumor immune microenvironment act reciprocally in driving tumor progression and diminishing the efficacy of cancer therapies. Extracellular vesicles (EVs), pivotal mediators of intercellular communications, build indispensable biological connections between CSCs and immune cells. By transferring bioactive molecules, including proteins, nucleic acids, and lipids, EVs can exert mutual influence on both CSCs and immune cells. This interaction plays a significant role in reshaping the tumor immune microenvironment, creating conditions favorable for the sustenance and propagation of CSCs. Deciphering the intricate interplay between CSCs and immune cells would provide valuable insights into the mechanisms of CSCs being more susceptible to immune escape. This review will highlight the EV-mediated communications between CSCs and each immune cell lineage in the tumor microenvironment and explore potential therapeutic opportunities.
Keywords: cancer stem cells; exosomes; extracellular vesicles; immune cells; tumor microenvironment.
Copyright © 2024 Li, Zhang, Yue and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cancer stem cells and tumor-associated macrophages as mates in tumor progression: mechanisms of crosstalk and advanced bioinformatic tools to dissect their phenotypes and interaction.Front Immunol. 2025 Feb 6;16:1529847. doi: 10.3389/fimmu.2025.1529847. eCollection 2025. Front Immunol. 2025. PMID: 39981232 Free PMC article. Review.
-
Exosome crosstalk between cancer stem cells and tumor microenvironment: cancer progression and therapeutic strategies.Stem Cell Res Ther. 2024 Nov 22;15(1):449. doi: 10.1186/s13287-024-04061-z. Stem Cell Res Ther. 2024. PMID: 39578849 Free PMC article. Review.
-
Interplay among extracellular vesicles, cancer stemness and immune regulation in driving hepatocellular carcinoma progression.Cancer Lett. 2024 Aug 10;597:217084. doi: 10.1016/j.canlet.2024.217084. Epub 2024 Jun 25. Cancer Lett. 2024. PMID: 38925362 Review.
-
Cancer Stem Cell Initiation by Tumor-Derived Extracellular Vesicles.Methods Mol Biol. 2022;2549:399-407. doi: 10.1007/7651_2021_371. Methods Mol Biol. 2022. PMID: 33755909
-
Challenges and opportunities for cancer stem cell-targeted immunotherapies include immune checkpoint inhibitor, cancer stem cell-dendritic cell vaccine, chimeric antigen receptor immune cells, and modified exosomes.J Biochem Mol Toxicol. 2024 Jun;38(6):e23719. doi: 10.1002/jbt.23719. J Biochem Mol Toxicol. 2024. PMID: 38764138 Review.
Cited by
-
Extracellular Vesicles in Ovarian Cancer: From Chemoresistance Mediators to Therapeutic Vectors.Biomedicines. 2024 Aug 9;12(8):1806. doi: 10.3390/biomedicines12081806. Biomedicines. 2024. PMID: 39200270 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

