Advances in gold catalyzed synthesis of quinoid heteroaryls
- PMID: 38962094
- PMCID: PMC11220603
- DOI: 10.1039/d4ra03368j
Advances in gold catalyzed synthesis of quinoid heteroaryls
Abstract
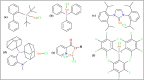
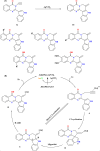
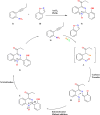
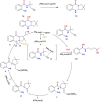
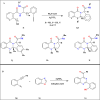
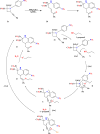
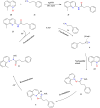
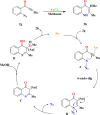
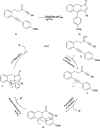
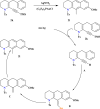
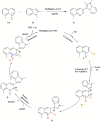
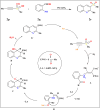
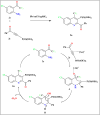
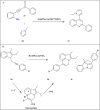
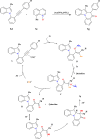
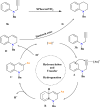
This review explores recent advancements in synthesizing quinoid heteroaryls, namely quinazoline and quinoline, vital in chemistry due to their prevalence in natural products and pharmaceuticals. It emphasizes the rapid, highly efficient, and economically viable synthesis achieved through gold-catalyzed cascade protocols. By investigating methodologies and reaction pathways, the review underscores exceptional yields attainable in the synthesis of quinoid heteroaryls. It offers valuable insights into accessing these complex structures through efficient synthetic routes. Various strategies, including cyclization, heteroarylation, cycloisomerization, cyclo-condensation, intermolecular and intramolecular cascade reactions, are covered, highlighting the versatility of gold-catalyzed approaches. The comprehensive compilation of different synthetic approaches and elucidation of reaction mechanisms contribute to a deeper understanding of the field. This review paves the way for future advancements in synthesizing quinoid heteroaryls and their applications in drug discovery and materials science.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Advancements in Gold-Catalyzed Cascade Reactions to Access Carbocycles and Heterocycles: An Overview.Chem Rec. 2023 Mar;23(3):e202200225. doi: 10.1002/tcr.202200225. Epub 2022 Dec 21. Chem Rec. 2023. PMID: 36543388 Review.
-
Synthetic routes to access dicarbonylated aryls and heteroaryls.Org Biomol Chem. 2024 Oct 23;22(41):8209-8248. doi: 10.1039/d4ob01278j. Org Biomol Chem. 2024. PMID: 39319402 Review.
-
Multicomponent Synthesis of Alkyl BCP-Heteroaryls via Electron Donor-Acceptor Complex Photoactivation under Mild Conditions.J Org Chem. 2025 Jan 31;90(4):1683-1696. doi: 10.1021/acs.joc.4c02941. Epub 2025 Jan 17. J Org Chem. 2025. PMID: 39818823
-
Recent Developments in Copper-Catalyzed Annulations for Synthesis of Spirooxindoles.Chem Rec. 2024 Nov;24(11):e202400126. doi: 10.1002/tcr.202400126. Epub 2024 Oct 22. Chem Rec. 2024. PMID: 39439210 Review.
-
Gold catalysis in quinoline synthesis.Chem Commun (Camb). 2024 Jul 4;60(55):6999-7016. doi: 10.1039/d4cc01915f. Chem Commun (Camb). 2024. PMID: 38904196 Review.
Cited by
-
Antibacterial Activity and Antifungal Activity of Monomeric Alkaloids.Toxins (Basel). 2024 Nov 12;16(11):489. doi: 10.3390/toxins16110489. Toxins (Basel). 2024. PMID: 39591244 Free PMC article. Review.
References
-
- Afzal O. Kumar S. Haider M. R. Ali M. R. Kumar R. Jaggi M. Bawa S. Eur. J. Med. Chem. 2015;97:871–910. - PubMed
-
- Selvam T. P. Kumar P. V. Res. Pharm. 2011;1:1–22.
-
- Selvam T. P. Kumar P. V. Res. J. Pharm. Technol. 2011;4:66–71.
-
- Alagarsamy V. Chitra K. Saravanan G. Solomon V. R. Sulthana M. T. Narendhar B. Eur. J. Med. Chem. 2018;151:628–685. - PubMed
-
- Alagarsamy V. Narendhar B. Chitra K. Sriram D. Sarvanan G. Solomon V. R. Russ. J. Bioorg. Chem. 2022;48:1221–1229.
Publication types
LinkOut - more resources
Full Text Sources

