Advances in the immunoescape mechanisms exploited by alphaherpesviruses
- PMID: 38962133
- PMCID: PMC11221368
- DOI: 10.3389/fmicb.2024.1392814
Advances in the immunoescape mechanisms exploited by alphaherpesviruses
Abstract
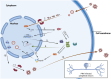
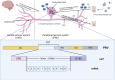
Alphaherpesviruses, categorized as viruses with linear DNA composed of two complementary strands, can potentially to induce diseases in both humans and animals as pathogens. Mature viral particles comprise of a core, capsid, tegument, and envelope. While herpesvirus infection can elicit robust immune and inflammatory reactions in the host, its persistence stems from its prolonged interaction with the host, fostering a diverse array of immunoescape mechanisms. In recent years, significant advancements have been achieved in comprehending the immunoescape tactics employed by alphaherpesviruses, including pseudorabies virus (PRV), herpes simplex virus (HSV), varicella-zoster virus (VZV), feline herpesvirus (FeHV), equine herpesvirus (EHV), and caprine herpesvirus type I (CpHV-1). Researchers have unveiled the intricate adaptive mechanisms existing between viruses and their natural hosts. This review endeavors to illuminate the research advancements concerning the immunoescape mechanisms of alphaherpesviruses by delineating the pertinent proteins and genes involved in virus immunity. It aims to furnish valuable insights for further research on related mechanisms and vaccine development, ultimately contributing to virus control and containment efforts.
Keywords: alphaherpesviruses; immune regulatory proteins; immunoescape; latency; signaling pathways.
Copyright © 2024 Wang, Ma, Wang, Wu, Chen, Ma, Wang, Qiu and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

