Isolation and identification of specific Enterococcus faecalis phage C-3 and G21-7 against Avian pathogenic Escherichia coli and its application to one-day-old geese
- PMID: 38962142
- PMCID: PMC11221357
- DOI: 10.3389/fmicb.2024.1385860
Isolation and identification of specific Enterococcus faecalis phage C-3 and G21-7 against Avian pathogenic Escherichia coli and its application to one-day-old geese
Abstract
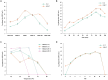
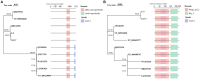
Colibacillosis caused by Avian pathogenic Escherichia coli (APEC), including peritonitis, respiratory tract inflammation and ovaritis, is recognized as one of the most common and economically destructive bacterial diseases in poultry worldwide. In this study, the characteristics and inhibitory potential of phages were investigated by double-layer plate method, transmission electron microscopy, whole genome sequencing, bioinformatics analysis and animal experiments. The results showed that phages C-3 and G21-7 isolated from sewage around goose farms infected multiple O serogroups (O1, O2, O18, O78, O157, O26, O145, O178, O103 and O104) Escherichia coli (E.coli) with a multiplicity of infection (MOI) of 10 and 1, respectively. According to the one-step growth curve, the incubation time of both bacteriophage C-3 and G21-7 was 10 min. Sensitivity tests confirmed that C-3 and G21-6 are stable at 4 to 50 °C and pH in the range of 4 to 11. Based on morphological and phylogenetic analysis, phages C-3 and G21-7 belong to Enterococcus faecalis (E. faecalis) phage species of the genus Saphexavirus of Herelleviridae family. According to genomic analysis, phage C-3 and G21-7 were 58,097 bp and 57,339 bp in size, respectively, with G+C content of 39.91% and 39.99%, encoding proteins of 97 CDS (105 to 3,993 bp) and 96 CDS (105 to 3,993 bp), and both contained 2 tRNAs. Both phages contained two tail proteins and holin-endolysin system coding genes, and neither carried resistance genes nor virulence factors. Phage mixture has a good safety profile and has shown good survival probability and feed efficiency in both treatment and prophylaxis experiments with one-day-old goslings. These results suggest that phage C-3 and G21-7 can be used as potential antimicrobials for the prevention and treatment of APEC.
Keywords: Avian pathogenic Escherichia coli; Enterococcus faecalis phage; genus Saphexavirus; one-day-old geese; virulent phage.
Copyright © 2024 Wang, Zhang, Zhang, Tong, Ma, Wang, Liu and Su.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The Isolation and Characterization of Bacteriophages Infecting Avian Pathogenic Escherichia coli O1, O2 and O78 Strains.Viruses. 2023 Oct 16;15(10):2095. doi: 10.3390/v15102095. Viruses. 2023. PMID: 37896873 Free PMC article.
-
Therapeutics and prophylactic efficacy of novel lytic Escherichia phage vB_EcoS_PJ16 against multidrug-resistant avian pathogenic E. coli using in vivo study.Int Microbiol. 2024 Jun;27(3):673-687. doi: 10.1007/s10123-023-00420-7. Epub 2023 Aug 26. Int Microbiol. 2024. PMID: 37632591
-
A lytic phage to control multidrug-resistant avian pathogenic Escherichia coli (APEC) infection.Front Cell Infect Microbiol. 2023 Sep 7;13:1253815. doi: 10.3389/fcimb.2023.1253815. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37743864 Free PMC article.
-
[Avian pathogenic Escherichia coli (APEC)].Berl Munch Tierarztl Wochenschr. 2003 Sep-Oct;116(9-10):381-95. Berl Munch Tierarztl Wochenschr. 2003. PMID: 14526468 Review. German.
-
Avian pathogenic Escherichia coli (APEC).Vet Res. 1999 Mar-Jun;30(2-3):299-316. Vet Res. 1999. PMID: 10367360 Review.
References
-
- Adorján A., Thuma Á., Könyves L., Tóth I. (2021). First isolation of atypical enteropathogenic Escherichia coli from geese (Anser anser domestica) and first description of atypical EPEC from turkeys and pigeons in Hungary. BMC Vet. Res. 17:263. doi: 10.1186/s12917-021-02968-w, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

