Case report and literature review: management of Paxlovid (nirmatrelvir/ritonavir)-induced acute tacrolimus toxicity in a patient with systemic lupus erythematosus
- PMID: 38962309
- PMCID: PMC11220238
- DOI: 10.3389/fphar.2024.1364121
Case report and literature review: management of Paxlovid (nirmatrelvir/ritonavir)-induced acute tacrolimus toxicity in a patient with systemic lupus erythematosus
Abstract
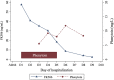
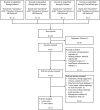
Despite the availability of effective vaccines and treatments for SARS-CoV-2, managing COVID-19 in patients with systemic lupus erythematosus (SLE) remains challenging, particularly considering drug-drug interactions (DDIs). Here, we present a case of DDIs between Tacrolimus (Tac) and nirmatrelvir/ritonavir (NMV/r) in a 32-year-old male with SLE. Following self-administration of NMV/r and resumption of Tac after 5 days, the patient experienced acute nephrotoxicity and neurotoxicity, accompanied by supratherapeutic Tac levels, despite Tac being withheld during NMV/r. The primary cause of this acute toxicity is attributed to ritonavir's inhibitory effect on both CYP3A4 enzymes and P-glycoprotein. Upon admission, Tac was discontinued, and supportive therapies were initiated. Phenytoin, a CYP3A4 inducer, was administered to lower Tac levels under the guidance of clinical pharmacists, effectively alleviating the patient's acute toxic symptoms. The half-life of Tac during the treatment of phenytoin was calculated to be 55.87 h. And no adverse reactions to phenytoin were observed. This case underscores the persistence of enzyme inhibition effects and demonstrates the effectiveness and safety of utilizing CYP3A4 enzyme inducers to mitigate Tac concentrations. Furthermore, it emphasizes the importance of healthcare providers and patients being vigilant about DDIs in Tac recipients. Lastly, it highlights the indispensable role of pharmacist involvement in clinical decision-making and close monitoring in complex clinical scenarios. Although our findings are based on a single case, they align with current knowledge and suggest the potential of individualized combination therapy in managing challenging COVID-19 cases in immunocompromised patients.
Keywords: CYP induction; case report; nirmatrelvir/ritonavir; systemic lupus erythematosus; tacrolimus.
Copyright © 2024 Jiang, Yan, Xia, Luo, Zheng, Tong, Liu, Zhu, Xu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A Case Report of Drug Interactions Between Nirmatrelvir/Ritonavir and Tacrolimus in a Patient With Systemic Lupus Erythematosus.Cureus. 2024 Jan 18;16(1):e52506. doi: 10.7759/cureus.52506. eCollection 2024 Jan. Cureus. 2024. PMID: 38371142 Free PMC article.
-
Case report: Paralytic ileus resulted from nirmatrelvir/ritonavir-tacrolimus drug-drug interaction in a systemic lupus erythematosus patient with COVID-19.Front Pharmacol. 2024 Mar 27;15:1389187. doi: 10.3389/fphar.2024.1389187. eCollection 2024. Front Pharmacol. 2024. PMID: 38601471 Free PMC article.
-
Influence of ensitrelvir or nirmatrelvir/ritonavir on tacrolimus clearance in kidney transplant recipients: a single-center case series.J Pharm Health Care Sci. 2024 Jul 10;10(1):37. doi: 10.1186/s40780-024-00361-x. J Pharm Health Care Sci. 2024. PMID: 38987842 Free PMC article.
-
Drug interactions between common dermatological medications and the oral anti-COVID-19 agents nirmatrelvir-ritonavir and molnupiravir.Ann Acad Med Singap. 2022 Dec;51(12):774-786. doi: 10.47102/annals-acadmedsg.2022289. Ann Acad Med Singap. 2022. PMID: 36592146
-
"Saving lives with nirmatrelvir/ritonavir one transplant patient at a time".Transpl Infect Dis. 2023 Apr;25(2):e14037. doi: 10.1111/tid.14037. Epub 2023 Feb 27. Transpl Infect Dis. 2023. PMID: 36847419 Free PMC article. Review.
References
-
- Akamatsu H., Kohno Y., Hashizume J., Nakagawa H., Kodama Y., Kawano H., et al. (2024). Effect of rifampicin administration on CYP induction in a dermatomyositis patient with vasospastic angina attributable to nilmatrelvir/ritonavir-induced blood tacrolimus elevation: a case report. J. Infect. Chemother., 10.1016/j.jiac.2024.02.006 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous