Dietary supplementation with a wild green oat extract (Avena sativa L.) to improve wellness and wellbeing during smoking reduction or cessation: a randomized double-blind controlled study
- PMID: 38962436
- PMCID: PMC11220258
- DOI: 10.3389/fnut.2024.1405156
Dietary supplementation with a wild green oat extract (Avena sativa L.) to improve wellness and wellbeing during smoking reduction or cessation: a randomized double-blind controlled study
Abstract
Objective: Smoking reduction or cessation are critical public health goals, given the well-documented risks of tobacco use to health. Reducing smoking frequency and cessation entirely are challenging due to nicotine addiction and withdrawal symptoms, which can significantly affect mental wellness and overall wellbeing. Previous research has suggested that certain dietary supplements may support smoking cessation and reduction efforts by mitigating these adverse effects. The objective of this study was to assess the effect of supplementation with 900 mg/day of Neuravena®, a green oat extract (GOE) of Avena sativa L., in enhancing wellness and wellbeing during a smoking reduction or cessation experience.
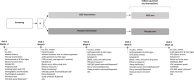
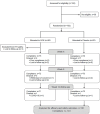
Methods: This was an 8-week randomized, double-blind, placebo-controlled study, ClinicalTrials Identifier: NCT04749017 (https://classic.clinicaltrials.gov/ct2/show/NCT04749017). Participants were assigned to one of the study groups, 72 participants were assigned to GOE and 73 to placebo. The subjects were followed for 8-weeks intervention period as well as an additional 4-week follow-up period. At subsequent visits, they underwent clinical assessments including assessments of quality of life, perceived stress, depression, nicotine dependence, anxiety, cognitive performance, and specific assessments of craving intensity.
Results: GOE was associated with greater improvements in elements of the abbreviated World Health Organization Quality of Life (WHOQOL-BREF) questionnaire as compared with placebo. Similar results were obtained from the SF-36 questionnaire and a visual QoL analogue scale (VAS). Perceived stress levels showed greater decline from baseline among the GOE supplemented participants as compared to placebo. Sleep quality parameters improved with GOE supplementation and worsened in the placebo group. At the end of the intervention period, the percentage of successful reducers (defined as >20% reduction in daily cigarettes) was higher in the GOE group as compared to placebo (66.7% vs. 49.3%, p = 0.034). The improvements from baseline in QoL measures in the GOE group persisted at 4 weeks after termination of the intervention.
Conclusion: GOE supplementation demonstrated greater improvements in quality of life measures, stress and sleep related parameters during a smoking reduction or cessation experience and the product was shown to be safe and well tolerated.
Keywords: Avena sativa; Neuravena®; dietary supplement; quality of life; tobacco.
Copyright © 2024 Friling, García-Muñoz, Lavie, Pérez-Piñero, Victoria-Montesinos, López-Román, García-Guillén, Muñoz-Carrillo, Cánovas, Ivanir and Jalanka.
Conflict of interest statement
MF, EI, AL, and JJ were employees of IFF Health, which commercializes the Neuravena®. FL-R, FC, AG-M, DV-M, SP-P, and AG-G were part of the Physiology Laboratory staff at Catholic University of Murcia, Murcia, Spain, and were hired by IFF Health to conduct the study. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Reitsma MB, Kendrick PJ, Ababneh E, Abbafati C, Abbasi-Kangevari M, Abdoli A, et al. . Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990†2019: a systematic analysis from the global burden of disease study 2019. Lancet. (2021) 397:2337–60. doi: 10.1016/S0140-6736(21)01169-7, PMID: - DOI - PMC - PubMed
-
- Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. (2010) 8:8. doi: 10.1186/1617-9625-8-8, PMID: - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

