Diagnostic accuracy of serum calprotectin measured by CLIA and EIA in juvenile idiopathic arthritis: a proof-of-concept study
- PMID: 38962573
- PMCID: PMC11219821
- DOI: 10.3389/fped.2024.1422916
Diagnostic accuracy of serum calprotectin measured by CLIA and EIA in juvenile idiopathic arthritis: a proof-of-concept study
Abstract
Objective: C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are used to assess disease activity in juvenile idiopathic arthritis (JIA). However, because these biomarkers do not always differentiate between active and inactive disease, there is a need for alternative markers such as serum calprotectin (sCal). The main aim of this proof-of-concept study was to assess the diagnostic accuracy of sCal in patients with JIA. Secondary aims were to identify the optimal sCal cut-off levels to define active disease and evaluate the association between these biomarkers and disease activity status.
Methods: Serum samples were obtained from 25 pediatric patients with JIA. Serum calprotectin levels were determined by two different assays, the QUANTA FLASH chemiluminescence immunoassay (CLIA) from Inova Diagnostics and the solid-phase enzyme immunoassay (EIA) from Bühlmann Laboratories. Diagnostic accuracy was assessed for sCal CLIA, sCal EIA, CRP, and ESR. The results obtained by the CLIA and EIA methodologies were compared. We also evaluated the association between the individual each biomarkers (sCal CLIA, sCal EIA, CRP, and ESR) and disease activity (according to JADAS-27 criteria and the ACR criteria modified by Anink and colleagues).
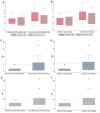
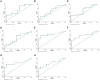
Results: For both sCal assays (CLIA and EIA), the optimal cut-off level (ROC analysis) was the same (2.3 µg/ml). Serum calprotectin levels measured by CLIA and EIA were strongly correlated with each other (Kendall's tau-b, 0.71; p < 0.001). Compared to ESR and CRP, sCal CLIA and EIA were both more accurate (i.e., greater sensitivity) in identifying patients with active disease. By contrast, ESR and CRP were more effective in identifying patients in remission (i.e., better specificity).
Conclusion: This proof-of-concept study shows that determination of serum calprotectin levels with CLIA or EIA can accurately identify the presence of active disease in patients with JIA.
Keywords: biomarkers; calcium-binding myeloid protein p8/14; juvenile idiopathic arthritis; s100 protein; s100A8/S100A9; serum calprotectin.
© 2024 Codes Méndez, Magallares-López, Park, Mariscal, Juárez, Boronat, Martínez-Martínez and Corominas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Serum calprotectin (S100A8/A9): a promising biomarker in diagnosis and follow-up in different subgroups of juvenile idiopathic arthritis.RMD Open. 2021 Jun;7(2):e001646. doi: 10.1136/rmdopen-2021-001646. RMD Open. 2021. PMID: 34108235 Free PMC article.
-
Serum Calprotectin Levels in Different Subtypes of Juvenile Idiopathic Arthritis (JIA) and Its Correlation with Quantitative CRP and JADAS-27.Indian J Pediatr. 2023 Dec;90(12):1177-1181. doi: 10.1007/s12098-022-04414-7. Epub 2023 Feb 10. Indian J Pediatr. 2023. PMID: 36763251
-
The Role of Serum Calprotectin in Defining Disease Outcomes in Non-Systemic Juvenile Idiopathic Arthritis: A Pilot Study.Int J Mol Sci. 2023 Jan 14;24(2):1671. doi: 10.3390/ijms24021671. Int J Mol Sci. 2023. PMID: 36675189 Free PMC article.
-
Serum Calprotectin a Potential Biomarker in Juvenile Idiopathic Arthritis: A Meta-Analysis.J Clin Med. 2021 Oct 22;10(21):4861. doi: 10.3390/jcm10214861. J Clin Med. 2021. PMID: 34768386 Free PMC article. Review.
-
From bench to bedside: Calprotectin (S100A8/S100A9) as a biomarker in rheumatoid arthritis.Front Immunol. 2022 Nov 3;13:1001025. doi: 10.3389/fimmu.2022.1001025. eCollection 2022. Front Immunol. 2022. PMID: 36405711 Free PMC article. Review.
References
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. (2004) 31(2):390–2. PMID: . - PubMed
-
- Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, pediatric rheumatology international trials organization international consensus. J Rheumatol. (2019) 46(2):190–7. 10.3899/jrheum.180168 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

