Advances in Bacterial Lysate Immunotherapy for Infectious Diseases and Cancer
- PMID: 38962577
- PMCID: PMC11221958
- DOI: 10.1155/2024/4312908
Advances in Bacterial Lysate Immunotherapy for Infectious Diseases and Cancer
Abstract
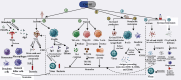
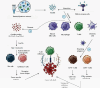
Antigenic cell fragments, pathogen-associated molecular patterns, and other immunostimulants in bacterial lysates or extracts may induce local and systemic immune responses in specific and nonspecific paradigms. Based on current knowledge, this review aimed to determine whether bacterial lysate has comparable functions in infectious diseases and cancer treatment. In infectious diseases, including respiratory and urinary tract infections, immune system activation by bacterial lysate can identify and combat pathogens. Commercially available bacterial lysates, including OM-85, Ismigen, Lantigen B, and LW 50020, were effective in children and adults in treating respiratory tract infections, chronic obstructive pulmonary disease, rhinitis, and rhinosinusitis with varying degrees of success. Moreover, OM-89, Uromune, Urovac, Urivac, and ExPEC4V showed therapeutic benefits in controlling urinary tract infections in adults, especially women. Bacterial lysate-based therapeutics are safe, well-tolerated, and have few side effects, making them a good alternative for infectious disease management. Furthermore, a nonspecific immunomodulation by bacterial lysates may stimulate innate immunity, benefiting cancer treatment. "Coley's vaccine" has been used to treat sarcomas, carcinomas, lymphomas, melanomas, and myelomas with varying outcomes. Later, several similar bacterial lysate-based therapeutics have been developed to treat cancers, including bladder cancer, non-small cell lung cancer, and myeloma; among them, BCG for in situ bladder cancer is well-known. Proinflammatory cytokines, including IL-1, IL-6, IL-12, and TNF-α, may activate bacterial antigen-specific adaptive responses that could restore tumor antigen recognition and response by tumor-specific type 1 helper cells and cytotoxic T cells; therefore, bacterial lysates are worth investigating as a vaccination adjuvants or add-on therapies for several cancers.
Copyright © 2024 Md. Mijanur Rahman et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Oral Bacterial Lysate OM-85: Advances in Pharmacology and Therapeutics.Drug Des Devel Ther. 2024 Oct 1;18:4387-4399. doi: 10.2147/DDDT.S484897. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39372675 Free PMC article. Review.
-
Bacterial immunostimulants--mechanism of action and clinical application in respiratory diseases.Pneumonol Alergol Pol. 2008;76(5):353-9. Pneumonol Alergol Pol. 2008. PMID: 19003766 Review.
-
Tumor cell lysates as immunogenic sources for cancer vaccine design.Hum Vaccin Immunother. 2014;10(11):3261-9. doi: 10.4161/21645515.2014.982996. Hum Vaccin Immunother. 2014. PMID: 25625929 Free PMC article. Review.
-
Applications of chemokines as adjuvants for vaccine immunotherapy.Immunobiology. 2018 Jun-Jul;223(6-7):477-485. doi: 10.1016/j.imbio.2017.12.001. Epub 2017 Dec 8. Immunobiology. 2018. PMID: 29246401 Free PMC article. Review.
-
Lysates of S. pyogenes serotype M49 induce pancreatic tumor growth delay by specific and unspecific antitumor immune responses.J Immunother. 2008 Oct;31(8):704-13. doi: 10.1097/CJI.0b013e3181829f62. J Immunother. 2008. PMID: 18779749
Cited by
-
Immunomodulatory Effects of Pulmonarom®: In Vitro Induction of TLR and Cytokine Expression in Human Dendritic Cells.Pharmaceuticals (Basel). 2025 Jun 13;18(6):885. doi: 10.3390/ph18060885. Pharmaceuticals (Basel). 2025. PMID: 40573281 Free PMC article.
-
Is There a Role for Immunostimulant Bacterial Lysates in the Management of Respiratory Tract Infection?Biomolecules. 2024 Oct 2;14(10):1249. doi: 10.3390/biom14101249. Biomolecules. 2024. PMID: 39456182 Free PMC article. Review.
-
Evaluation of Immunostimulatory Effects of Bacterial Lysate Proteins on THP-1 Macrophages: Pro-inflammatory Cytokine Response and Proteomic Profiling.J Immunol Res. 2025 Apr 25;2025:2289241. doi: 10.1155/jimr/2289241. eCollection 2025. J Immunol Res. 2025. PMID: 40322557 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

