Dramatic Radiographic Response of Pelvis-Filling Locally Advanced Cervical Cancer Treated With Radiation and Chemotherapy
- PMID: 38962615
- PMCID: PMC11219247
- DOI: 10.7759/cureus.61544
Dramatic Radiographic Response of Pelvis-Filling Locally Advanced Cervical Cancer Treated With Radiation and Chemotherapy
Abstract
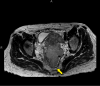
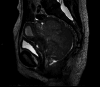
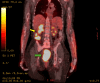
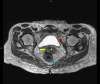
Locally advanced cervical cancers are often treated with palliative intent due to concerns that the tumor is too far advanced or too large to be treated curatively. Also, patients greater than 65 years of age with cervical cancer are sometimes regarded as being too old or too frail to be cured with combined radiation and chemotherapy. These patients are often treated with radiation alone or with palliative therapy. Understanding the treatment modalities for cervical cancer is essential, as they can be complex and unique to each patient's specific diagnosis. This case report aims to describe the dramatic response to treatment with combined radiation and chemotherapy for a patient greater than 65 years of age with pelvis-filling cervical cancer with right-sided hydronephrosis. After a five-week course of concurrent chemoradiation, the cervical mass radiographically completely disappeared, with no evidence of disease noted on pelvic MRI.
Keywords: concurrent chemoradiation therapy; gynecology-oncology; hdr (high dose rate) brachytherapy; intensity modulated radiation therapy (imrt); locally advanced cervical cancer.
Copyright © 2024, Bedi et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


Similar articles
-
The prognostic factors for locally advanced cervical cancer patients treated by intensity-modulated radiation therapy with concurrent chemotherapy.J Formos Med Assoc. 2015 Mar;114(3):231-7. doi: 10.1016/j.jfma.2012.10.021. Epub 2013 Jan 5. J Formos Med Assoc. 2015. PMID: 25777974
-
Concurrent Chemo- Radiobrachytherapy with Cisplatin and Medium Dose Rate Intra- Cavitary Brachytherapy for Locally Advanced Uterine Cervical Cancer.Asian Pac J Cancer Prev. 2018 Oct 26;19(10):2745-2750. doi: 10.22034/APJCP.2018.19.10.2745. Asian Pac J Cancer Prev. 2018. PMID: 30360600 Free PMC article. Clinical Trial.
-
Management of refractory metastatic anal squamous cell carcinoma following disease progression on traditional chemoradiation therapy.J Adv Pract Oncol. 2012 May;3(3):161-9. doi: 10.6004/jadpro.2012.3.3.4. J Adv Pract Oncol. 2012. PMID: 25031942 Free PMC article.
-
Optimal management of locally advanced cervical carcinoma.J Natl Cancer Inst Monogr. 1996;(21):89-92. J Natl Cancer Inst Monogr. 1996. PMID: 9023835 Review.
-
Why brachytherapy boost is the treatment of choice for most women with locally advanced cervical carcinoma?Brachytherapy. 2016 Mar-Apr;15(2):191-9. doi: 10.1016/j.brachy.2015.12.003. Epub 2016 Feb 1. Brachytherapy. 2016. PMID: 26810408 Review.
References
-
- A review of cervical cancer: incidence and disparities. Buskwofie A, David-West G, Clare CA. J Natl Med Assoc. 2020;112:229–232. - PubMed
-
- U.S. Cancer Statistics Working Group. U.S. cancer statistics data visualizations tool, based on 2022 submission data (1999-2020). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. 2022. https://www.cdc.gov/cancer/dataviz https://www.cdc.gov/cancer/dataviz
-
- Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): a randomised, double-blind, phase 3 clinical trial. Lorusso D, Xiang Y, Kosei Hasegawa, et al. https://pubmed.ncbi.nlm.nih.gov/38521086/ Lancet. 2024;403:0–50. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
