Initial therapeutic evidence of a borosilicate bioactive glass (BSG) and Fe3O4 magnetic nanoparticle scaffold on implant-associated Staphylococcal aureus bone infection
- PMID: 38962659
- PMCID: PMC11220464
- DOI: 10.1016/j.bioactmat.2024.05.040
Initial therapeutic evidence of a borosilicate bioactive glass (BSG) and Fe3O4 magnetic nanoparticle scaffold on implant-associated Staphylococcal aureus bone infection
Abstract
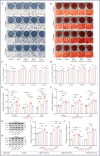
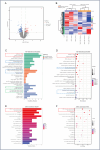
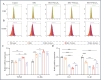
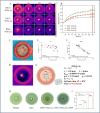
Implant-associated Staphylococcus aureus (S. aureus) osteomyelitis is a severe challenge in orthopedics. While antibiotic-loaded bone cement is a standardized therapeutic approach for S. aureus osteomyelitis, it falls short in eradicating Staphylococcus abscess communities (SACs) and bacteria within osteocyte-lacuna canalicular network (OLCN) and repairing bone defects. To address limitations, we developed a borosilicate bioactive glass (BSG) combined with ferroferric oxide (Fe3O4) magnetic scaffold to enhance antibacterial efficacy and bone repair capabilities. We conducted comprehensive assessments of the osteoinductive, immunomodulatory, antibacterial properties, and thermal response of this scaffold, with or without an alternating magnetic field (AMF). Utilizing a well-established implant-related S. aureus tibial infection rabbit model, we evaluated its antibacterial performance in vivo. RNA transcriptome sequencing demonstrated that BSG + 5%Fe3O4 enhanced the immune response to bacteria and promoted osteogenic differentiation and mineralization of MSCs. Notably, BSG + 5%Fe3O4 upregulated gene expression of NOD-like receptor and TNF pathway in MSCs, alongside increased the expression of osteogenic factors (RUNX2, ALP and OCN) in vitro. Flow cytometry on macrophage exhibited a polarization effect towards M2, accompanied by upregulation of anti-inflammatory genes (TGF-β1 and IL-1Ra) and downregulation of pro-inflammatory genes (IL-6 and IL-1β) among macrophages. In vivo CT imaging revealed the absence of osteolysis and periosteal response in rabbits treated with BSG + 5%Fe3O4 + AMF at 42 days. Histological analysis indicated complete controls of SACs and bacteria within OLCN by day 42, along with new bone formation, signifying effective control of S. aureus osteomyelitis. Further investigations will focus on the in vivo biosafety and biological mechanism of this scaffold within infectious microenvironment.
Keywords: Biofilm; Borosilicate bioactive glass; Magnetic nanoparticles; Osteomyelitis; Staphylococcus aureus.
© 2024 The Authors.
Conflict of interest statement
Haobo Pan is an editorial board member for Biomedical Technology and was not involved in the editorial review or the decision to publish this article. All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Ren Y., Weeks J., Xue T., et al. Evidence of bisphosphonate-conjugated sitafloxacin eradication of established methicillin-resistant S. aureus infection with osseointegration in murine models of implant-associated osteomyelitis. Bone Res. 2023;11(1):51. doi: 10.1038/s41413-023-00287-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

