Characterization of the effects of cannabinoid receptor deletion on energy metabolism in female C57BL mice
- PMID: 38962676
- PMCID: PMC11221337
- DOI: 10.3389/fendo.2024.1386230
Characterization of the effects of cannabinoid receptor deletion on energy metabolism in female C57BL mice
Abstract
Background: Despite the evidence that energy balance is regulated differently in females and that the endocannabinoid system is sexually dimorphic, previous studies on the endocannabinoid system and energy balance predominantly used male models. Here, we characterize the effects of cannabinoid receptor deletion on body weight gain and glucose metabolism in female C57BL mice.
Methods: Female mice lacking the cannabinoid-1 receptor (CB1R-/-), cannabinoid-2 receptor (CB2R-/-), or both receptors (CB1R-/-/CB2R-/-) and wild-type (WT) mice were fed with a low (LFD; 10% of calories from fat) or high-fat diet (HFD; 45% of calories from fat) for six weeks.
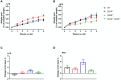
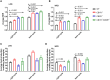
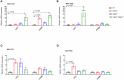
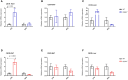
Results: Female WT mice fed with HFD gained significantly more weight than WT mice fed with LFD (p < 0.001). Similar pattern was observed for CB2/- mice fed with HFD compared to CB2R-/- mice fed with LFD (p < 0.001), but not for CB1R-/- fed with HFD vs. LFD (p = 0.22) or CB1R-/-/CB2R-/- fed with HFD vs. LFD (p = 0.96). Comparing the 4 groups on LFD, weight gain of CB1R-/- mice was greater than all other genotypes (p < 0.05). When fed with HFD, the deletion of CB1R alone in females did not attenuate weight gain compared to WT mice (p = 0.72). Female CB1R-/-/CB2R-/- mice gained less weight than WT mice when fed with HFD (p = 0.007) despite similar food intake and locomotor activity, potentially owing to enhanced thermogenesis in the white adipose tissue. No significant difference in weight gain was observed for female CB2R-/- and WT mice on LFD or HFD. Fasting glucose, however, was higher in CB2R-/- mice fed with LFD than all other groups (p < 0.05).
Conclusion: The effects of cannabinoid receptor deletion on glucose metabolism in female mice were similar to previously published findings on male mice, yet the effects on body weight gain and thermogenesis were attenuated in CB1R-/- mice.
Keywords: body weight; cannabinoids; female; glucose metabolism; thermogenesis.
Copyright © 2024 Sotzen, Ahmed, Olson and Alshaarawy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Low Basal CB2R in Dopamine Neurons and Microglia Influences Cannabinoid Tetrad Effects.Int J Mol Sci. 2020 Dec 21;21(24):9763. doi: 10.3390/ijms21249763. Int J Mol Sci. 2020. PMID: 33371336 Free PMC article.
-
Diet-Induced Obesity in Cannabinoid-2 Receptor Knockout Mice and Cannabinoid Receptor 1/2 Double-Knockout Mice.Obesity (Silver Spring). 2019 Mar;27(3):454-461. doi: 10.1002/oby.22403. Epub 2019 Jan 30. Obesity (Silver Spring). 2019. PMID: 30699233 Free PMC article.
-
Expression of the cannabinoid system in muscle: effects of a high-fat diet and CB1 receptor blockade.Biochem J. 2011 Jan 1;433(1):175-85. doi: 10.1042/BJ20100751. Biochem J. 2011. PMID: 20955176
-
Understanding the possible role of endocannabinoid system in obesity.Prostaglandins Other Lipid Mediat. 2021 Feb;152:106520. doi: 10.1016/j.prostaglandins.2020.106520. Epub 2020 Nov 26. Prostaglandins Other Lipid Mediat. 2021. PMID: 33249225 Review.
-
Revealing the therapeutic potential of synthetic cannabinoids: a systematic review of cannabinoid receptor binding dynamics and their implications for cancer therapy.J Cannabis Res. 2025 Jun 7;7(1):33. doi: 10.1186/s42238-025-00289-5. J Cannabis Res. 2025. PMID: 40483537 Free PMC article. Review.
References
-
- Substance Abuse and Mental Health Services Administration . Center for Behavioral Health Statistics and Quality. Rockville, MD: Substance Abuse and Mental Health Services Administration. (2020). Vol. (HHS Publication No. PEP20-07-01-001, NSDUH Series H-55).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

