Therapeutic implications for sphingolipid metabolism in metabolic dysfunction-associated steatohepatitis
- PMID: 38962680
- PMCID: PMC11220194
- DOI: 10.3389/fendo.2024.1400961
Therapeutic implications for sphingolipid metabolism in metabolic dysfunction-associated steatohepatitis
Abstract
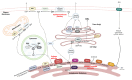
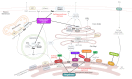
The prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD), a leading cause of chronic liver disease, has increased worldwide along with the epidemics of obesity and related dysmetabolic conditions characterized by impaired glucose metabolism and insulin signaling, such as type 2 diabetes mellitus (T2D). MASLD can be defined as an excessive accumulation of lipid droplets in hepatocytes that occurs when the hepatic lipid metabolism is totally surpassed. This metabolic lipid inflexibility constitutes a central node in the pathogenesis of MASLD and is frequently linked to the overproduction of lipotoxic species, increased cellular stress, and mitochondrial dysfunction. A compelling body of evidence suggests that the accumulation of lipid species derived from sphingolipid metabolism, such as ceramides, contributes significantly to the structural and functional tissue damage observed in more severe grades of MASLD by triggering inflammatory and fibrogenic mechanisms. In this context, MASLD can further progress to metabolic dysfunction-associated steatohepatitis (MASH), which represents the advanced form of MASLD, and hepatic fibrosis. In this review, we discuss the role of sphingolipid species as drivers of MASH and the mechanisms involved in the disease. In addition, given the absence of approved therapies and the limited options for treating MASH, we discuss the feasibility of therapeutic strategies to protect against MASH and other severe manifestations by modulating sphingolipid metabolism.
Keywords: hepatic fibrosis; inflammation; lipotoxicity; mitochondrial dysfunction; sphingolipids; steatohepatitis; steatotic liver.
Copyright © 2024 Ramos-Molina, Rossell, Pérez-Montes de Oca, Pardina, Genua, Rojo-López, Julián, Alonso, Julve and Mauricio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Le P, Payne JY, Zhang L, Deshpande A, Rothberg MB, Alkhouri N, et al. . Disease state transition probabilities across the spectrum of NAFLD: A systematic review and meta-analysis of paired biopsy or imaging studies. Clin Gastroenterol Hepatol. (2023) 21:1154–68. doi: 10.1016/j.cgh.2022.07.033 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

