Non-infectious immune complexes downregulate the production of interferons and tumor necrosis factor-α in primary porcine alveolar macrophages in vitro
- PMID: 38962699
- PMCID: PMC11221350
- DOI: 10.3389/fvets.2024.1420466
Non-infectious immune complexes downregulate the production of interferons and tumor necrosis factor-α in primary porcine alveolar macrophages in vitro
Abstract
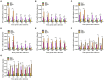
Porcine reproductive and respiratory syndrome (PRRS) caused by the PRRS virus (PRRSV) has been harming the pig industry worldwide for nearly 40 years. Although scientific researchers have made substantial efforts to explore PRRSV pathogenesis, the immune factors influencing PRRSV infection still need to be better understood. Infectious virus-antibody immune complexes (ICs) formed by PRRSV and sub-or non-neutralizing antibodies specific for PRRSV may significantly promote the development of PRRS by enhancing PRRSV replication through antibody-dependent enhancement. However, nothing is known about whether PRRSV infection is affected by non-infectious ICs (NICs) formed by non-pathogenic/infectious antigens and corresponding specific antibodies. Here, we found that PRRSV significantly induced the transcripts and proteins of interferon-α (IFN-α), IFN-β, IFN-γ, IFN-λ1, and tumor necrosis factor-α (TNF-α) in vitro primary porcine alveolar macrophages (PAMs) in the early stage of infection. Our results showed that NICs formed by rabbit-negative IgG (RNI) and pig anti-RNI specific IgG significantly reduced the transcripts and proteins of IFN-α, IFN-β, IFN-γ, IFN-λ1, and TNF-α in vitro PAMs and significantly elevated the transcripts and proteins of interleukine-10 (IL-10) and transforming growth factor-β1 (TGF-β1) in vitro PAMs. NICs-mediated PRRSV infection showed that NICs not only significantly decreased the induction of IFN-α, IFN-β, IFN-γ, IFN-λ1, and TNF-α by PRRSV but also significantly increased the induction of IL-10 and TGF-β1 by PRRSV and considerably enhanced PRRSV replication in vitro PAMs. Our data suggested that NICs could downregulate the production of antiviral cytokines (IFN-α/β/γ/λ1 and TNF-α) during PRRSV infection in vitro and facilitated PRRSV proliferation in its host cells by inhibiting innate antiviral immune response. This study elucidated one novel immune response to PRRSV infection, which would enhance our understanding of the pathogenesis of PRRSV.
Keywords: IFNs; NICS; PAMS; PRRSV; TNF-α.
Copyright © 2024 Zhang, Feng, Chen, Wang, He, Fan and Liu.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Activation of activating Fc gamma receptors down-regulates the levels of interferon β, interferon γ and interferon λ1 in porcine alveolar macrophages during PRRSV infection.Int Immunopharmacol. 2020 Apr;81:106268. doi: 10.1016/j.intimp.2020.106268. Epub 2020 Feb 13. Int Immunopharmacol. 2020. PMID: 32062072
-
Antibody-dependent enhancement of porcine reproductive and respiratory syndrome virus infection downregulates the levels of interferon-gamma/lambdas in porcine alveolar macrophages in vitro.Front Vet Sci. 2023 Mar 15;10:1150430. doi: 10.3389/fvets.2023.1150430. eCollection 2023. Front Vet Sci. 2023. PMID: 37008366 Free PMC article.
-
Identification of the RNA Pseudoknot within the 3' End of the Porcine Reproductive and Respiratory Syndrome Virus Genome as a Pathogen-Associated Molecular Pattern To Activate Antiviral Signaling via RIG-I and Toll-Like Receptor 3.J Virol. 2018 May 29;92(12):e00097-18. doi: 10.1128/JVI.00097-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29618647 Free PMC article.
-
Antibody-Mediated Porcine Reproductive and Respiratory Syndrome Virus Infection Downregulates the Production of Interferon-α and Tumor Necrosis Factor-α in Porcine Alveolar Macrophages via Fc Gamma Receptor I and III.Viruses. 2020 Feb 8;12(2):187. doi: 10.3390/v12020187. Viruses. 2020. PMID: 32046249 Free PMC article.
-
Antagonizing interferon-mediated immune response by porcine reproductive and respiratory syndrome virus.Biomed Res Int. 2014;2014:315470. doi: 10.1155/2014/315470. Epub 2014 Jul 3. Biomed Res Int. 2014. PMID: 25101271 Free PMC article. Review.
References
-
- Yim-Im W, Huang H, Park J, Wang C, Calzada G, Gauger P, et al. . Comparison of ZMAC and MARC-145 cell lines for improving porcine reproductive and respiratory Ssyndrome virus isolation from clinical samples. J Clin Microbiol. (2021) 59:e01757–20. doi: 10.1128/JCM.01757-20, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

