Plasma concentrations of buprenorphine administered via matrix-type transdermal patches applied at three different anatomical locations in healthy adult horses
- PMID: 38962712
- PMCID: PMC11220193
- DOI: 10.3389/fpain.2024.1390322
Plasma concentrations of buprenorphine administered via matrix-type transdermal patches applied at three different anatomical locations in healthy adult horses
Abstract
Background: Anatomical location-dependent differences in transdermal opioid penetration are well described in human patients. Although this has been investigated in horses with fentanyl, there is no literature available on location-dependent plasma buprenorphine concentrations when administered as a transdermal matrix-type patch.
Objective: This study aims to compare the plasma concentrations achieved from the matrix-type transdermal buprenorphine patches placed at different anatomical sites (metacarpus, gaskin, and ventral tail base) in healthy adult horses.
Study design: This is a randomized experimental study with a Latin square design.
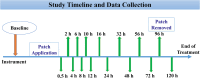
Methods: Six adult horses were given each of three treatments with a minimum 10-day washout period. For each treatment, two 20 μg h-1 matrix-type buprenorphine patches were applied to the ventral aspect of the tail base (TailTDP), metacarpus region (MetacarpusTDP), or gaskin region (GaskinTDP). Whole blood samples (for determination of buprenorphine concentration) and physiological variables were collected before (0 h) and at 0.5, 2, 4, 6, 8, 10, 12, 16, 24, 32, 48, 56, 72, 96 and 120 h after patches were applied. The patches were removed 96 h following placement and were analyzed for residual buprenorphine content. Buprenorphine concentrations were measured in plasma by LC-MS/MS. A mixed-effects model was used to analyze the physiological variables.
Results: Between the three treatment groups, there was no change in physiological variables across timepoints as compared to baseline and when compared to each other in a single horse and between horses (p > 0.3). When comparing all three locations, the buprenorphine uptake was observed to be more consistent with respect to measurable plasma concentrations >0.1 ng ml-1 when applied to the ventral aspect of the tail base. In the TailTDP group, the mean plasma buprenorphine concentrations were >0.1 ng ml-1 from 2 to 32 h. The highest group mean was 0.25 ng ml-1 noted at 4 h.
Conclusions: The metacarpal and gaskin regions presented more erratic and inconsistent buprenorphine uptake and plasma concentrations as compared to the ventral aspect of the tail base. Further research must be directed at investigating the optimal dose, achievable duration of analgesia, change in measurable plasma concentrations, and behavioral and systemic effects.
Keywords: analgesia; equine; gaskin; metacarpus; opioids; pain; pharmacology; tail base.
© 2024 Paranjape, Knych, Berghaus, Giancola, Cathcart and Reed.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Evaluation of physical variables, thermal nociceptive threshold testing and pharmacokinetics during placement of transdermal buprenorphine matrix-type patch in healthy adult horses.Front Pain Res (Lausanne). 2024 Mar 11;5:1373555. doi: 10.3389/fpain.2024.1373555. eCollection 2024. Front Pain Res (Lausanne). 2024. PMID: 38529072 Free PMC article.
-
The pharmacokinetics of a fentanyl matrix patch applied at three different anatomical locations in horses.Equine Vet J. 2022 Jan;54(1):153-158. doi: 10.1111/evj.13424. Epub 2021 Mar 10. Equine Vet J. 2022. PMID: 33453066
-
Pharmacokinetics, pharmacodynamics and antinociceptive effects of buprenorphine following transdermal administration to horses.Vet Anaesth Analg. 2024 Sep-Oct;51(5):520-530. doi: 10.1016/j.vaa.2024.05.001. Epub 2024 May 10. Vet Anaesth Analg. 2024. PMID: 38834387
-
Transdermal buprenorphine in the treatment of chronic pain.Expert Rev Neurother. 2005 May;5(3):315-23. doi: 10.1586/14737175.5.3.315. Expert Rev Neurother. 2005. PMID: 15938664 Review.
-
Buprenorphine 5, 10 and 20 μg/h transdermal patch: a review of its use in the management of chronic non-malignant pain.Drugs. 2011 Dec 24;71(18):2491-509. doi: 10.2165/11208250-000000000-00000. Drugs. 2011. PMID: 22141389 Review.

