Evidence for inflammation in normal-appearing brain regions in patients with growing sporadic vestibular schwannoma: A PET study
- PMID: 38962752
- PMCID: PMC11221070
- DOI: 10.1093/noajnl/vdae094
Evidence for inflammation in normal-appearing brain regions in patients with growing sporadic vestibular schwannoma: A PET study
Abstract
Background: Nonauditory symptoms can be a prominent feature in patients with sporadic vestibular schwannoma (VS), but the cause of these symptoms is unknown. Inflammation is hypothesized to play a key role in the growth and symptomatic presentation of sporadic VS, and in this study, we investigated through translocator protein (TSPO) positron emission tomography (PET) whether inflammation occurred within the "normal appearing" brain of such patients and its association with tumor growth.
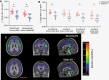
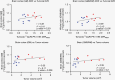
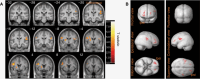
Methods: Dynamic PET datasets from 15 patients with sporadic VS (8 static and 7 growing) who had been previously imaged using the TSPO tracer [11C](R)-PK11195 were included. Parametric images of [11C](R)-PK11195 binding potential (BPND) and the distribution volume ratio (DVR) were derived and compared across VS growth groups within both contralateral and ipsilateral gray (GM) and white matter (WM) regions. Voxel-wise cluster analysis was additionally performed to identify anatomical regions of increased [11C](R)-PK11195 binding.
Results: Compared with static tumors, growing VS demonstrated significantly higher cortical (GM, 1.070 vs. 1.031, P = .03) and whole brain (GM & WM, 1.045 vs. 1.006, P = .03) [11C](R)-PK11195 DVR values. The voxel-wise analysis supported the region-based analysis and revealed clusters of high TSPO binding within the precentral, postcentral, and prefrontal cortex in patients with growing VS.
Conclusions: We present the first in vivo evidence of increased TSPO expression and inflammation within the brains of patients with growing sporadic VS. These results provide a potential mechanistic insight into the development of nonauditory symptoms in these patients and highlight the need for further studies interrogating the role of neuroinflammation in driving VS symptomatology.
Keywords: PET; TSPO; inflammation; microglia; vestibular schwannoma.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Inflammation and vascular permeability correlate with growth in sporadic vestibular schwannoma.Neuro Oncol. 2019 Feb 19;21(3):314-325. doi: 10.1093/neuonc/noy177. Neuro Oncol. 2019. PMID: 30388263 Free PMC article.
-
[¹¹C]-(R)PK11195 tracer kinetics in the brain of glioma patients and a comparison of two referencing approaches.Eur J Nucl Med Mol Imaging. 2013 Sep;40(9):1406-19. doi: 10.1007/s00259-013-2447-2. Epub 2013 May 29. Eur J Nucl Med Mol Imaging. 2013. PMID: 23715902 Free PMC article.
-
In Vivo Detection of Diffuse Inflammation in Secondary Progressive Multiple Sclerosis Using PET Imaging and the Radioligand ¹¹C-PK11195.J Nucl Med. 2014 Jun;55(6):939-44. doi: 10.2967/jnumed.113.131698. Epub 2014 Apr 7. J Nucl Med. 2014. PMID: 24711650
-
Innate immune cells and myelin profile in multiple sclerosis: a multi-tracer PET/MR study.Eur J Nucl Med Mol Imaging. 2022 Nov;49(13):4551-4566. doi: 10.1007/s00259-022-05899-2. Epub 2022 Jul 15. Eur J Nucl Med Mol Imaging. 2022. PMID: 35838758
-
Sifting through the surfeit of neuroinflammation tracers.J Cereb Blood Flow Metab. 2018 Feb;38(2):204-224. doi: 10.1177/0271678X17748786. Epub 2017 Dec 19. J Cereb Blood Flow Metab. 2018. PMID: 29256293 Free PMC article. Review.
Cited by
-
Emerging strategies for the prediction of behaviour, growth, and treatment response in vestibular schwannoma.Acta Neurochir (Wien). 2025 Apr 22;167(1):116. doi: 10.1007/s00701-025-06522-7. Acta Neurochir (Wien). 2025. PMID: 40261443 Free PMC article. Review.
-
Hemispheric asymmetry of [11C](R)PK11195 binding to translocator protein 18 kDa (TSPO) in normal brain.J Cereb Blood Flow Metab. 2025 Jun 19:271678X251348790. doi: 10.1177/0271678X251348790. Online ahead of print. J Cereb Blood Flow Metab. 2025. PMID: 40536168 Free PMC article.
-
Brain structural alterations in vestibular schwannoma beyond tinnitus and hearing loss.Brain Commun. 2025 Mar 11;7(2):fcaf107. doi: 10.1093/braincomms/fcaf107. eCollection 2025. Brain Commun. 2025. PMID: 40144300 Free PMC article.
References
-
- Sagers JE, Sahin MI, Moon I, et al.. NLRP3 inflammasome activation in human vestibular schwannoma: implications for tumor-induced hearing loss. Hear Res. 2019;381(9):107770. - PubMed
LinkOut - more resources
Full Text Sources
