Heukharang (Lactuca sativa L.) extracts enhanced the sleep behavior of mice: potential involvement of adenosine A1 and A2A receptors
- PMID: 38962793
- PMCID: PMC11217248
- DOI: 10.1007/s41105-024-00522-3
Heukharang (Lactuca sativa L.) extracts enhanced the sleep behavior of mice: potential involvement of adenosine A1 and A2A receptors
Abstract
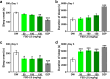
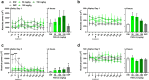
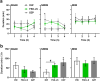
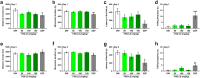
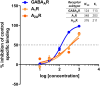
A significant proportion of the world's population suffers from insomnia, a disorder characterized by complications in initiating and maintaining sleep. Many medications used to treat insomnia target the γ-aminobutyric acid (GABA) neurotransmitter system. However, these substances, such as benzodiazepines, induce significant adverse consequences, including dependence and memory impairment, after prolonged use. Thus, current studies are aimed at developing therapeutic hypnotics derived from natural sources that may cause less severe side effects. Heukharang is a variety of lettuce from Korea that was discovered to contain sleep-promoting compounds. Therefore, we investigated the potential effects of sub-chronic administration of Heukharang extract (FSD-LS) on sleep behavior (pentobarbital-induced sleeping test), brain wave activity and sleep architecture (electroencephalography), and physiological behavior (open-field test and rota-rod) in mice, along with radioligand binding assays (GABAA, adenosine A1 and A2A receptors). We found that FSD-LS prolonged the total sleep duration and reduced the onset time of sleep, and enhanced delta wave power and non-rapid eye movement (NREM) sleep duration, all indicating persistent sleep-enhancing effects. FSD-LS lacked adverse effects on the spontaneous locomotor activity and motor coordination of mice, unlike diazepam. Pharmacological blocking using caffeine and bicuculline supported the possible involvement of adenosine receptors in the sleep-promoting effects of FSD-LS, with partial contribution from GABA receptor activity. Overall, our study recommends FSD-LS as a potential source for the development of sleep-aiding therapeutics.
Keywords: Adenosine A1 receptor; Adenosine A2A receptor; EEG; FSD-LS; NREM; Sleep.
© The Author(s), under exclusive licence to Japanese Society of Sleep Research 2024. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors have no conflicts of interest to declare.
Figures
Similar articles
-
Extracts of Prunella vulgaris Enhanced Pentobarbital-Induced Sleeping Behavior in Mice Potentially via Adenosine A2A Receptor Activity.Planta Med. 2024 Sep;90(11):864-875. doi: 10.1055/a-2360-9639. Epub 2024 Jul 24. Planta Med. 2024. PMID: 39047773
-
Heukharang lettuce (Lactuca sativa L.) leaf extract displays sleep-promoting effects through GABAA receptor.J Ethnopharmacol. 2023 Oct 5;314:116602. doi: 10.1016/j.jep.2023.116602. Epub 2023 May 4. J Ethnopharmacol. 2023. PMID: 37149068
-
Sleep-enhancing effect of Hongcheon-hop (Humulus lupulus L.) extract containing xanthohumol and humulone through GABAA receptor.J Ethnopharmacol. 2025 Feb 10;338(Pt 2):119019. doi: 10.1016/j.jep.2024.119019. Epub 2024 Nov 9. J Ethnopharmacol. 2025. PMID: 39522843
-
Uridine receptor: discovery and its involvement in sleep mechanism.Sleep. 2001 May 1;24(3):251-60. doi: 10.1093/sleep/24.3.251. Sleep. 2001. PMID: 11322706 Review.
-
Adenosine A2A receptors and sleep.Int Rev Neurobiol. 2023;170:155-178. doi: 10.1016/bs.irn.2023.04.007. Epub 2023 Apr 29. Int Rev Neurobiol. 2023. PMID: 37741690 Review.
Cited by
-
Foods for Sleep Improvement: A Review of the Potential and Mechanisms Involved.Foods. 2025 Mar 21;14(7):1080. doi: 10.3390/foods14071080. Foods. 2025. PMID: 40238208 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
