Cytokine responses of CD4+ T cells and NKT cells to periodontitis-associated bacteria in individuals with or without periodontitis
- PMID: 38962877
- PMCID: PMC11873674
- DOI: 10.1111/jre.13317
Cytokine responses of CD4+ T cells and NKT cells to periodontitis-associated bacteria in individuals with or without periodontitis
Abstract
Aim: Periodontitis is an inflammatory disease driven by opportunistic bacteria including Porphyromonas gingivalis and Fusobacterium nucleatum, where T-cell and NKT-cell responses to these bacteria in patients with periodontitis grade B or C are not fully elucidated. The objective is to determine if exaggerated proinflammatory Th-cell responses to periodontitis-associated bacteria, but not commensal bacteria, is a characteristic of increased periodontitis grade.
Methods: Mononuclear cells from patients with periodontitis grade C (n = 26) or grade B (n = 33) and healthy controls (HCs; n = 26) were stimulated with P. gingivalis, F. nucleatum or the commensal bacteria, Staphylococcus epidermidis and Cutibacterium acnes. Cytokine production by different T-cell populations and FOXP3-expression by regulatory T cells were assessed by flow cytometry.
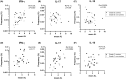
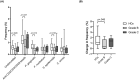
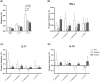
Results: Compared to HCs, grade C patients had decreased frequencies of interleukin (IL)-10-producing CD4+ T cells before stimulation (p = .02) and increased frequencies of IFN-y-producing CD4+ T cells after stimulation with P. gingivalis (p = .0019). Grade B patients had decreased frequencies of FOXP3+ CD4+ T cells before (p = .030) before and after stimulation with anti-CD2/anti-CD3/anti-CD28-loaded beads (p = .047), P. gingivalis (p = .013) and S. epidermidis (p = .018). Clinical attachment loss correlated with the frequencies of IFN-y-producing Th1 cells in P. gingivalis- and F. nucleatum-stimulated cultures in grade B patients (p = .023 and p = .048, respectively) and with the frequencies of Th17 cells in P. gingivalis-stimulated cultures (p = .0062) in grade C patients. Patients with periodontitis grade C or grade B showed lower frequencies of IL-10-producing NKT cells than HCs in unstimulated cultures (p = .0043 and p = .027 respectively).
Conclusions: Both periodontitis groups showed decreased frequencies of immunoregulatory T-cell and NKT cell subsets at baseline. Clinical attachment loss correlated with P. gingivalis-induced Th17-responses in grade C patients and with Th1-responses in grade B patients when cells were stimulated with P. gingivalis, supporting that dysregulated pro-inflammatory T-cell responses to periodontitis-associated bacteria contribute to the pathogenesis of periodontitis.
Keywords: Fusobacterium Nucleatum; Porphyromonas gingivalis; NKT cells; T‐helper cells; cytokines; periodontitis.
© 2024 The Author(s). Journal of Periodontal Research published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare no financial conflicts of interest.
Figures

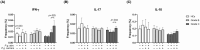
Similar articles
-
B-cell cytokine responses to Porphyromonas gingivalis in patients with periodontitis and healthy controls.J Periodontol. 2023 Aug;94(8):997-1007. doi: 10.1002/JPER.22-0438. Epub 2023 Mar 5. J Periodontol. 2023. PMID: 36715211
-
Different responses in B cells induced by Porphyromonas gingivalis and Fusobacterium nucleatum.Arch Oral Biol. 1992;37(7):565-73. doi: 10.1016/0003-9969(92)90139-y. Arch Oral Biol. 1992. PMID: 1359860
-
Effect of Fusobacterium nucleatum on the T and B cell responses to Porphyromonas gingivalis in a mouse model.Clin Exp Immunol. 2002 May;128(2):238-44. doi: 10.1046/j.1365-2249.2002.01852.x. Clin Exp Immunol. 2002. PMID: 11985513 Free PMC article.
-
About a Possible Impact of Endodontic Infections by Fusobacterium nucleatum or Porphyromonas gingivalis on Oral Carcinogenesis: A Literature Overview.Int J Mol Sci. 2024 May 7;25(10):5083. doi: 10.3390/ijms25105083. Int J Mol Sci. 2024. PMID: 38791123 Free PMC article. Review.
-
Regulatory B and T cell responses in patients with autoimmune thyroid disease and healthy controls.Dan Med J. 2016 Feb;63(2):B5177. Dan Med J. 2016. PMID: 26836805 Review.
References
-
- Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Investig. 1976;34(3):235‐249. - PubMed
-
- Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and Peri‐implant diseases and conditions. J Clin Periodontol. 2018;45(Suppl 20):S162‐S170. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

