Zinc Promoted Cross-Electrophile Sulfonylation to Access Alkyl-Alkyl Sulfones
- PMID: 38962907
- PMCID: PMC11347995
- DOI: 10.1002/advs.202406228
Zinc Promoted Cross-Electrophile Sulfonylation to Access Alkyl-Alkyl Sulfones
Abstract
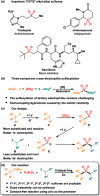
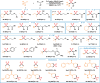
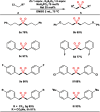
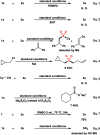
The transition metal-catalyzed multi-component cross-electrophile sulfonylation, which incorporates SO2 as a linker within organic frameworks, has proven to be a powerful, efficient, and cost-effective means of synthesizing challenging alkyl-alkyl sulfones. Transition metal catalysts play a crucial role in this method by transferring electrons from reductants to electrophilic organohalides, thereby causing undesirable side reactions such as homocoupling, protodehalogenation, β-hydride elimination, etc. It is worth noting that tertiary alkyl halides have rarely been demonstrated to be compatible with current methods owing to various undesired side reactions. In this work, a zinc-promoted cross-electrophile sulfonylation is developed through a radical-polar crossover pathway. This approach enables the synthesis of various alkyl-alkyl sulfones, including 1°-1°, 2°-1°, 3°-1°, 2°-2°, and 3°-2° types, from inexpensive and readily available alkyl halides. Various functional groups are well tolerated in the work, resulting in yields of up to 93%. Additionally, this protocol has been successfully applied to intramolecular sulfonylation and homo-sulfonylation reactions. The insights gained from this work shall be useful for the further development of cross-electrophile sulfonylation to access alkyl-alkyl sulfones.
Keywords: alkyl–alkyl sulfone; catalyst‐free; cross‐electrophile coupling; organic halides; sulfonylation.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- a) Wang J., Hou T., J. Chem. Inf. Model. 2009, 50, 55; - PubMed
- b) Xu W.‐M., Han F.‐F., He M., Hu D.‐Y., He J., Yang S., Song B.‐A., J. Agric. Food Chem. 2012, 60, 1036; - PubMed
- c) Ilardi E. A., Vitaku E., Njardarson J. T., J. Med. Chem. 2013, 57, 2832; - PubMed
- d) Scott K. A., Njardarson J. T., Top. Curr. Chem 2018, 376, 5; - PubMed
- e) Wang N., Saidhareddy P., Jiang X., Nat. Prod. Rep. 2020, 37, 246. - PubMed
-
- a) Carmine A. A., Brogden R. N., Heel R. C., Speight T. M., Avery G. S., Drugs 1982, 24, 85; - PubMed
- b) Liu K. K. C., Bailey S., Dinh D. M., Lam H., Li C., Wells P. A., Yin M.‐J., Zou A., Bioorg. Med. Chem. Lett. 2012, 22, 5114; - PubMed
- c) Rueeger H., Lueoend R., Rogel O., Rondeau J.‐M., Möbitz H., Machauer R., Jacobson L., Staufenbiel M., Desrayaud S., Neumann U., J. Med. Chem. 2012, 55, 3364; - PubMed
- d) Deeks E. D., Drugs 2020, 80, 181. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
