Dendritic Cell-Hitchhiking In Vivo for Vaccine Delivery to Lymph Nodes
- PMID: 38962939
- PMCID: PMC11434131
- DOI: 10.1002/advs.202402199
Dendritic Cell-Hitchhiking In Vivo for Vaccine Delivery to Lymph Nodes
Abstract
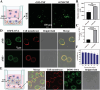
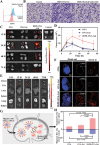
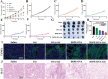
Therapeutic cancer vaccines are among the first FDA-approved cancer immunotherapies. Among them, it remains a major challenge to achieve robust lymph-node (LN) accumulation. However, delivering cargo into LN is difficult owing to the unique structure of the lymphatics, and clinical responses have been largely disappointing. Herein, inspired by the Migrated-DCs homing from the periphery to the LNs, an injectable hydrogel-based polypeptide vaccine system is described for enhancing immunostimulatory efficacy, which could form a local niche of vaccine "hitchhiking" on DCs. The OVA peptide modified by lipophilic DSPE domains in the hydrogel is spontaneously inserted into the cell membrane to achieve "antigen anchoring" on DCs in vivo. Overall, OVA peptide achieves active access LNs through recruiting and "hitchhiking" subcutaneous Migrated-DCs. Remarkably, it is demonstrated that the composite hydrogel enhances LNs targeting efficacy by approximately six-fold compared to free OVA peptide. Then, OVA peptide can be removed from the cell surface under a typical acidic microenvironment within the LNs, further share them with LN-resident APCs via the "One-to-Many" strategy (One Migrated-DC corresponding to Many LN-resident APCs), thereby activating powerful immune stimulation. Moreover, the hydrogel vaccine exhibits significant tumor growth inhibition in melanoma and inhibits pulmonary metastatic nodule formation.
Keywords: dendritic cell‐hitchhiking; lymph node targeting; vaccine delivery.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Phenotypic profile of dendritic and T cells in the lymph node of Balb/C mice with breast cancer submitted to dendritic cells immunotherapy.Immunol Lett. 2016 Sep;177:25-37. doi: 10.1016/j.imlet.2016.07.009. Epub 2016 Jul 14. Immunol Lett. 2016. PMID: 27423825
-
Delivered antigen peptides to resident CD8α+ DCs in lymph node by micelle-based vaccine augment antigen-specific CD8+ effector T cell response.Eur J Pharm Biopharm. 2020 Feb;147:76-86. doi: 10.1016/j.ejpb.2019.12.013. Epub 2019 Dec 28. Eur J Pharm Biopharm. 2020. PMID: 31887349
-
Construction of lymph nodes-targeting tumor vaccines by using the principle of DNA base complementary pairing to enhance anti-tumor cellular immune response.J Nanobiotechnology. 2024 May 8;22(1):230. doi: 10.1186/s12951-024-02498-1. J Nanobiotechnology. 2024. PMID: 38720322 Free PMC article.
-
Migration of dendritic cells to the lymph nodes and its enhancement to drive anti-tumor responses.Crit Rev Oncol Hematol. 2016 Nov;107:100-110. doi: 10.1016/j.critrevonc.2016.09.002. Epub 2016 Sep 9. Crit Rev Oncol Hematol. 2016. PMID: 27823637 Review.
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
Cited by
-
Targeted Delivery of Personalized Cancer Vaccines Based on Antibody-Antigen Complexes.Vaccines (Basel). 2025 Mar 19;13(3):324. doi: 10.3390/vaccines13030324. Vaccines (Basel). 2025. PMID: 40266219 Free PMC article.
-
Bioinspired micro- and nanostructured systems for cancer therapy.MedComm (2020). 2024 Nov 28;5(12):e70025. doi: 10.1002/mco2.70025. eCollection 2024 Dec. MedComm (2020). 2024. PMID: 39619230 Free PMC article. Review.
-
Nonviral targeted mRNA delivery: principles, progresses, and challenges.MedComm (2020). 2025 Jan 2;6(1):e70035. doi: 10.1002/mco2.70035. eCollection 2025 Jan. MedComm (2020). 2025. PMID: 39760110 Free PMC article. Review.
-
TGF-β Decreases NK Cell Mobility and Cytotoxic Efficacy in Complex in vitro Models of the Leukemia Microenvironment.Immunotargets Ther. 2025 Jun 18;14:589-604. doi: 10.2147/ITT.S512700. eCollection 2025. Immunotargets Ther. 2025. PMID: 40546335 Free PMC article.
References
-
- Newman J. H., Chesson C. B., Herzog N. L., Bommareddy P. K., Aspromonte S. M., Pepe R., Estupinian R., Aboelatta M. M., Buddhadev S., Tarabichi S., Lee M., Li S., Medina D. J., Giurini E. F., Gupta K. H., Guevara‐Aleman G., Rossi M., Nowicki C., Abed A., Goldufsky J. W., Broucek J. R., Redondo R. E., Rotter D., Jhawar S. R., Wang S. J., Kohlhapp F. J., Kaufman H. L., Thomas P. G., Gupta V., Kuzel T. M., Proc Natl Acad Sci U S A. 2020, 117, 1119. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
