piRNA PROPER Suppresses DUSP1 Translation by Targeting N6-Methyladenosine-Mediated RNA Circularization to Promote Oncogenesis of Prostate Cancer
- PMID: 38962952
- PMCID: PMC11434016
- DOI: 10.1002/advs.202402954
piRNA PROPER Suppresses DUSP1 Translation by Targeting N6-Methyladenosine-Mediated RNA Circularization to Promote Oncogenesis of Prostate Cancer
Abstract
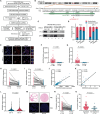
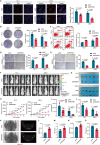
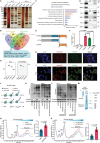
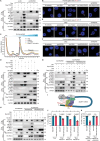
Genetic and epigenetic alterations occur in many physiological and pathological processes. The existing knowledge regarding the association of PIWI-interacting RNAs (piRNAs) and their genetic variants on risk and progression of prostate cancer (PCa) is limited. In this study, three genome-wide association study datasets are combined, including 85,707 PCa cases and 166,247 controls, to uncover genetic variants in piRNAs. Functional investigations involved manipulating piRNA expression in cellular and mouse models to study its oncogenetic role in PCa. A specific genetic variant, rs17201241 is identified, associated with increased expression of PROPER (piRNA overexpressed in prostate cancer) in tumors and are located within the gene, conferring an increased risk and malignant progression of PCa. Mechanistically, PROPER coupled with YTHDF2 to recognize N6-methyladenosine (m6A) and facilitated RNA-binding protein interactions between EIF2S3 at 5'-untranslated region (UTR) and YTHDF2/YBX3 at 3'-UTR to promote DUSP1 circularization. This m6A-dependent mRNA-looping pattern enhanced DUSP1 degradation and inhibited DUSP1 translation, ultimately reducing DUSP1 expression and promoting PCa metastasis via the p38 mitogen-activated protein kinase (MAPK) signaling pathway. Inhibition of PROPER expression using antagoPROPER effectively suppressed xenograft growth, suggesting its potential as a therapeutic target. Thus, targeting piRNA PROPER-mediated genetic and epigenetic fine control is a promising strategy for the concurrent prevention and treatment of PCa.
Keywords: N6‐methyladenosine; PIWI‐interacting RNA; Prostate cancer; Single nucleotide polymorphism.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Ca‐Cancer J. Clin. 2021, 71, 209. - PubMed
-
- Gao P., Xia J.‐H., Sipeky C., Dong X.‐M., Zhang Q., Yang Y., Zhang P., Cruz S. P., Zhang K., Zhu J., Lee H.‐M., Suleman S., Giannareas N., Liu S., Tammela T. L. J., Auvinen A., Wang X., Huang Q., Wang L., Manninen A., Vaarala M. H., Wang L., Schleutker J., Wei G.‐H., Cell. 2018, 174, P576. - PMC - PubMed
-
- Hua J. T., Ahmed M., Guo H., Zhang Y., Chen S., Soares F., Lu J., Zhou S., Wang M., Li H., Larson N. B., McDonnell S. K., Patel P. S., Liang Y., Yao C. Q., van der Kwast T., Lupien M., Feng F. Y., Zoubeidi A., Tsao M.‐S., Thibodeau S. N., Boutros P. C., He H. H., Cell. 2018, 174, P564. - PubMed
-
- Jiang X., Guo S., Wang S., Zhang Y., Chen H., Wang Y., Liu R., Niu Y., Xu Y., Cancer Res. 2022, 82, 831. - PubMed
-
- Chen S., Huang V., Xu X., Livingstone J., Soares F., Jeon J., Zeng Y., Hua J. T., Petricca J., Guo H., Wang M., Yousif F., Zhang Y., Donmez N., Ahmed M., Volik S., Lapuk A., Chua M. L. K., Heisler L. E., Foucal A., Fox N. S., Fraser M., Bhandari V., Shiah Y.‐J., Guan J., Li J., Orain M., Picard V., Hovington H., Bergeron A., et al., Cell. 2019, 176, P831. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
