The intracellular domain of Sema6A is essential for development of the zebrafish retina
- PMID: 38963001
- PMCID: PMC11795297
- DOI: 10.1242/jcs.261469
The intracellular domain of Sema6A is essential for development of the zebrafish retina
Abstract
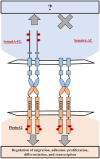
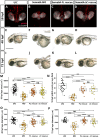
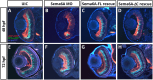
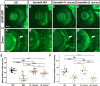
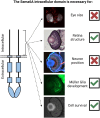
Semaphorin6A (Sema6A) is a repulsive guidance molecule that plays many roles in central nervous system, heart and bone development, as well as immune system responses and cell signaling in cancer. Loss of Sema6A or its receptor PlexinA2 in zebrafish leads to smaller eyes and improper retinal patterning. Here, we investigate a potential role for the Sema6A intracellular domain in zebrafish eye development and dissect which phenotypes rely on forward signaling and which rely on reverse signaling. We performed rescue experiments on zebrafish Sema6A morphants with either full-length Sema6A (Sema6A-FL) or Sema6A lacking its intracellular domain (Sema6A-ΔC). We identified that the intracellular domain is not required for eye size and retinal patterning, however it is required for retinal integrity, the number and end feet strength of Müller glia and protecting against retinal cell death. This novel function for the intracellular domain suggests a role for Sema6A reverse signaling in zebrafish eye development.
Keywords: Eye development; Retina; Reverse signaling; Semaphorin; Zebrafish.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

