Evaluation of inpatient and emergency department healthcare resource utilization and costs pre- and post-nusinersen for the treatment of spinal muscular atrophy using United States claims
- PMID: 38963060
- PMCID: PMC11225157
- DOI: 10.57264/cer-2023-0187
Evaluation of inpatient and emergency department healthcare resource utilization and costs pre- and post-nusinersen for the treatment of spinal muscular atrophy using United States claims
Abstract
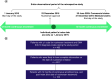
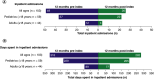
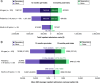
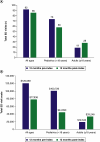
Aim: Nusinersen, administered by intrathecal injection at a dose of 12 mg, is indicated across all ages for the treatment of spinal muscular atrophy (SMA). Evidence on real-world healthcare resource use (HRU) and costs among patients taking nusinersen remains limited. This study aimed to evaluate real-world HRU and costs associated with nusinersen use through US claims databases. Patients & methods: Using the Merative™ MarketScan® Research Databases, patients with SMA receiving nusinersen were identified from commercial (January 2017 to June 2020) and Medicaid claims (January 2017 to December 2019). Those likely to have complete information on the date of nusinersen initiation and continuous enrollment 12 months pre- and post-index (first record of nusinersen treatment) were retained. Number and costs (US$ 2020) of inpatient admissions and emergency department (ED) visits, unrelated to nusinersen administration, were evaluated for 12 months pre- and post-nusinersen initiation and stratified by age: pediatric (<18 years) and adult (≥18 years). Results: Overall, 103 individuals treated with nusinersen were retained: 59 were pediatric (mean age [range]: 9 [1-17] years), and 44 were adults (30 [18-63] years). Inpatient admissions decreased by 41% for pediatrics and 67% for adults in the 12 months post-treatment versus the 12 months pre-treatment. Average inpatient admission costs per patient for the pediatric cohort decreased by 63% ($22,903 vs $8466) and by 79% ($13,997 vs $2899) for the adult cohort when comparing the 12 months pre-index with the 12 months post-index period. Total ED visits and ED visit costs decreased by 8% and 35%, respectively, for the overall cohort over the 12-month period pre- and post-index. Conclusion: Using US claims databases, nusinersen treatment in pediatric and adult patients was associated with reductions in HRU and costs over a 12-month period post-treatment initiation relative to the pre-treatment period.
Keywords: commercial and Medicaid claims; healthcare resource utilization; medical cost offsets; nusinersen; patient-level claims; spinal muscular atrophy.
Conflict of interest statement
Competing interests disclosure
The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
Similar articles
-
Health Care Resource Utilization and Costs for Patients with Spinal Muscular Atrophy: Findings from a Retrospective US Claims Database Analysis.Adv Ther. 2023 Oct;40(10):4589-4605. doi: 10.1007/s12325-023-02621-y. Epub 2023 Aug 16. Adv Ther. 2023. PMID: 37587305 Free PMC article.
-
Economic burden of spinal muscular atrophy in the United States: a contemporary assessment.J Med Econ. 2020 Jan;23(1):70-79. doi: 10.1080/13696998.2019.1646263. Epub 2019 Aug 4. J Med Econ. 2020. PMID: 31322019
-
Examining Real-World Adherence to Nusinersen for the Treatment of Spinal Muscular Atrophy Using Two Large US Data Sources.Adv Ther. 2023 Mar;40(3):1129-1140. doi: 10.1007/s12325-022-02414-9. Epub 2023 Jan 16. Adv Ther. 2023. PMID: 36645543 Free PMC article.
-
Nusinersen for Spinal Muscular Atrophy in the United States: Findings From a Retrospective Claims Database Analysis.Adv Ther. 2021 Dec;38(12):5809-5828. doi: 10.1007/s12325-021-01938-w. Epub 2021 Oct 28. Adv Ther. 2021. PMID: 34713391 Free PMC article.
-
Drug treatment for spinal muscular atrophy type I.Cochrane Database Syst Rev. 2019 Dec 11;12(12):CD006281. doi: 10.1002/14651858.CD006281.pub5. Cochrane Database Syst Rev. 2019. PMID: 31825542 Free PMC article.
References
-
- Mercuri E, Sumner CJ, Muntoni F, Darras BT, Finkel RS. Spinal muscular atrophy. Nat. Rev. Dis. Primers 8(1), 52 (2022). - PubMed
-
- Darras BT, Monani UR, De Vivo DC. Genetic disorders affecting the motor neuron: spinal muscular atrophy. In: Swaiman's Pediatric Neurology (6th Edition). Swaiman KF, Ashwal S, Ferriero DMet al.. et al. (Eds). Elsevier, The Netherlands, 1057–1064 (2017).
-
- Hoy SM. Nusinersen: first global approval. Drugs 77(4), 473–479 (2017). - PubMed
LinkOut - more resources
Full Text Sources
