Novel Insights into the Catalytic Mechanism of Collagenolysis by Zn(II)-Dependent Matrix Metalloproteinase-1
- PMID: 38963231
- PMCID: PMC11309001
- DOI: 10.1021/acs.biochem.4c00076
Novel Insights into the Catalytic Mechanism of Collagenolysis by Zn(II)-Dependent Matrix Metalloproteinase-1
Abstract
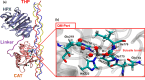
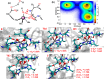
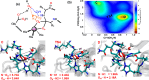
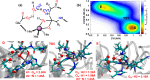
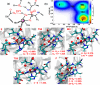
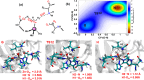
Collagen hydrolysis, catalyzed by Zn(II)-dependent matrix metalloproteinases (MMPs), is a critical physiological process. Despite previous computational investigations into the catalytic mechanisms of MMP-mediated collagenolysis, a significant knowledge gap in understanding remains regarding the influence of conformational sampling and entropic contributions at physiological temperature on enzymatic collagenolysis. In our comprehensive multilevel computational study, employing quantum mechanics/molecular mechanics (QM/MM) metadynamics (MetD) simulations, we aimed to bridge this gap and provide valuable insights into the catalytic mechanism of MMP-1. Specifically, we compared the full enzyme-substrate complex in solution, clusters in solution, and gas-phase to elucidate insights into MMP-1-catalyzed collagenolysis. Our findings reveal significant differences in the catalytic mechanism when considering thermal effects and the dynamic evolution of the system, contrasting with conventional static potential energy surface QM/MM reaction path studies. Notably, we observed a significant stabilization of the critical tetrahedral intermediate, attributed to contributions from conformational flexibility and entropy. Moreover, we found that protonation of the scissile bond nitrogen occurs via proton transfer from a Zn(II)-coordinated hydroxide rather than from a solvent water molecule. Following C-N bond cleavage, the C-terminus remains coordinated to the catalytic Zn(II), while the N-terminus forms a hydrogen bond with a solvent water molecule. Subsequently, the release of the C-terminus is facilitated by the coordination of a water molecule. Our study underscores the pivotal role of protein conformational dynamics at physiological temperature in stabilizing the transition state of the rate-limiting step and key intermediates, compared to the corresponding reaction in solution. These fundamental insights into the mechanism of collagen degradation provide valuable guidance for the development of MMP-1-specific inhibitors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Quantum Mechanics/Molecular Mechanics Simulations Distinguish Insulin-Regulated Aminopeptidase Substrate (Oxytocin) and Inhibitor (Angiotensin IV) and Reveal Determinants of Activity and Inhibition.J Chem Inf Model. 2025 Jun 23;65(12):6261-6272. doi: 10.1021/acs.jcim.5c00869. Epub 2025 Jun 11. J Chem Inf Model. 2025. PMID: 40495656 Free PMC article.
-
Reaction Mechanism and Metal Selectivity of Human SAMHD1 Elucidated by QM/MM Calculations.ACS Catal. 2025 Jun 1;15(12):10176-10187. doi: 10.1021/acscatal.5c01682. eCollection 2025 Jun 20. ACS Catal. 2025. PMID: 40568220 Free PMC article.
-
Catalytic Mechanism of Collagen Hydrolysis by Zinc(II)-Dependent Matrix Metalloproteinase-1.J Phys Chem B. 2023 Nov 16;127(45):9697-9709. doi: 10.1021/acs.jpcb.3c04293. Epub 2023 Nov 6. J Phys Chem B. 2023. PMID: 37931179 Free PMC article.
-
Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults.Cochrane Database Syst Rev. 2016 Apr 21;4(4):CD009016. doi: 10.1002/14651858.CD009016.pub2. Cochrane Database Syst Rev. 2016. PMID: 27098439 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

