Interleukin-5 alleviates cardiac remodelling via the STAT3 pathway in angiotensin II-infused mice
- PMID: 38963241
- PMCID: PMC11223166
- DOI: 10.1111/jcmm.18493
Interleukin-5 alleviates cardiac remodelling via the STAT3 pathway in angiotensin II-infused mice
Abstract
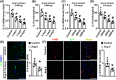
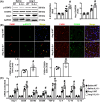
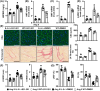
Interleukin-5 (IL-5) has been reported to be involved in cardiovascular diseases, such as atherosclerosis and cardiac injury. This study aimed to investigate the effects of IL-5 on cardiac remodelling. Mice were infused with angiotensin II (Ang II), and the expression and source of cardiac IL-5 were analysed. The results showed that cardiac IL-5 expression was time- and dose-dependently decreased after Ang II infusion, and was mainly derived from cardiac macrophages. Additionally, IL-5-knockout (IL-5-/-) mice were used to observe the effects of IL-5 knockout on Ang II-induced cardiac remodelling. We found knockout of IL-5 significantly increased the expression of cardiac hypertrophy markers, elevated myocardial cell cross-sectional areas and worsened cardiac dysfunction in Ang II-infused mice. IL-5 deletion also promoted M2 macrophage differentiation and exacerbated cardiac fibrosis. Furthermore, the effects of IL-5 deletion on cardiac remodelling was detected after the STAT3 pathway was inhibited by S31-201. The effects of IL-5 on cardiac remodelling and M2 macrophage differentiation were reversed by S31-201. Finally, the effects of IL-5 on macrophage differentiation and macrophage-related cardiac hypertrophy and fibrosis were analysed in vitro. IL-5 knockout significantly increased the Ang II-induced mRNA expression of cardiac hypertrophy markers in myocardial cells that were co-cultured with macrophages, and this effect was reversed by S31-201. Similar trends in the mRNA levels of fibrosis markers were observed when cardiac fibroblasts and macrophages were co-cultured. In conclusions, IL-5 deficiency promote the differentiation of M2 macrophages by activating the STAT3 pathway, thereby exacerbating cardiac remodelling in Ang II-infused mice. IL-5 may be a potential target for the clinical prevention of cardiac remodelling.
Keywords: STAT3 pathway; angiotensin II; cardiac remodelling; interleukin‐5; macrophage differentiation.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures




Similar articles
-
CD1d-dependent natural killer T cells attenuate angiotensin II-induced cardiac remodelling via IL-10 signalling in mice.Cardiovasc Res. 2019 Jan 1;115(1):83-93. doi: 10.1093/cvr/cvy164. Cardiovasc Res. 2019. PMID: 29939225
-
CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration.Eur Heart J. 2018 May 21;39(20):1818-1831. doi: 10.1093/eurheartj/ehy085. Eur Heart J. 2018. PMID: 29514257
-
SGK3 deficiency in macrophages suppresses angiotensin II-induced cardiac remodeling via regulating Ndufa13-mediated mitochondrial oxidative stress.Cell Mol Life Sci. 2024 Aug 19;81(1):359. doi: 10.1007/s00018-024-05395-w. Cell Mol Life Sci. 2024. PMID: 39158709 Free PMC article.
-
Pathophysiology of Angiotensin II-Mediated Hypertension, Cardiac Hypertrophy, and Failure: A Perspective from Macrophages.Cells. 2024 Dec 4;13(23):2001. doi: 10.3390/cells13232001. Cells. 2024. PMID: 39682749 Free PMC article. Review.
-
Macrophages in Cardiovascular Fibrosis: Novel Subpopulations, Molecular Mechanisms, and Therapeutic Targets.Can J Cardiol. 2025 Feb;41(2):309-322. doi: 10.1016/j.cjca.2024.11.018. Epub 2024 Nov 22. Can J Cardiol. 2025. PMID: 39580052 Review.
References
-
- Herrmann M, Taban‐Shomal O, Hübner U, Böhm M, Herrmann W. A review of homocysteine and heart failure. Eur J Heart Fail. 2006;8(6):571‐576. - PubMed
-
- Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119(1):159‐176. - PubMed
-
- Sekaran NK, Crowley AL, de Souza FR, Resende ES, Rao SV. The role for cardiovascular remodeling in cardiovascular outcomes. Curr Atheroscler Rep. 2017;19:23. - PubMed
-
- Kumar S, Wang G, Zheng N, et al. HIMF (hypoxia‐induced mitogenic factor)‐IL (interleukin)‐6 signaling mediates cardiomyocyte‐fibroblast crosstalk to promote cardiac hypertrophy and fibrosis. Hypertension. 2019;73(5):1058‐1070. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

