Mechanisms of microbial co-aggregation in mixed anaerobic cultures
- PMID: 38963458
- PMCID: PMC11224092
- DOI: 10.1007/s00253-024-13246-8
Mechanisms of microbial co-aggregation in mixed anaerobic cultures
Abstract
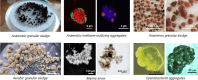
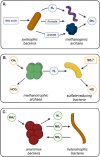
Co-aggregation of anaerobic microorganisms into suspended microbial biofilms (aggregates) serves ecological and biotechnological functions. Tightly packed aggregates of metabolically interdependent bacteria and archaea play key roles in cycling of carbon and nitrogen. Additionally, in biotechnological applications, such as wastewater treatment, microbial aggregates provide a complete metabolic network to convert complex organic material. Currently, experimental data explaining the mechanisms behind microbial co-aggregation in anoxic environments is scarce and scattered across the literature. To what extent does this process resemble co-aggregation in aerobic environments? Does the limited availability of terminal electron acceptors drive mutualistic microbial relationships, contrary to the commensal relationships observed in oxygen-rich environments? And do co-aggregating bacteria and archaea, which depend on each other to harvest the bare minimum Gibbs energy from energy-poor substrates, use similar cellular mechanisms as those used by pathogenic bacteria that form biofilms? Here, we provide an overview of the current understanding of why and how mixed anaerobic microbial communities co-aggregate and discuss potential future scientific advancements that could improve the study of anaerobic suspended aggregates. KEY POINTS: • Metabolic dependency promotes aggregation of anaerobic bacteria and archaea • Flagella, pili, and adhesins play a role in the formation of anaerobic aggregates • Cyclic di-GMP/AMP signaling may trigger the polysaccharides production in anaerobes.
Keywords: Anaerobic biofilm; Cell-to-cell adhesion; Co-aggregation; Exopolysaccharides; Syntrophy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Community Composition and Ultrastructure of a Nitrate-Dependent Anaerobic Methane-Oxidizing Enrichment Culture.Appl Environ Microbiol. 2018 Jan 17;84(3):e02186-17. doi: 10.1128/AEM.02186-17. Print 2018 Feb 1. Appl Environ Microbiol. 2018. PMID: 29150508 Free PMC article.
-
Establishing anaerobic hydrocarbon-degrading enrichment cultures of microorganisms under strictly anoxic conditions.Nat Protoc. 2018 Jun;13(6):1310-1330. doi: 10.1038/nprot.2018.030. Epub 2018 May 17. Nat Protoc. 2018. PMID: 29773905
-
Heterotrophic archaea contribute to carbon cycling in low-pH, suboxic biofilm communities.Appl Environ Microbiol. 2012 Dec;78(23):8321-30. doi: 10.1128/AEM.01938-12. Epub 2012 Sep 21. Appl Environ Microbiol. 2012. PMID: 23001646 Free PMC article.
-
Exocellular electron transfer in anaerobic microbial communities.Environ Microbiol. 2006 Mar;8(3):371-82. doi: 10.1111/j.1462-2920.2006.00989.x. Environ Microbiol. 2006. PMID: 16478444 Review.
-
Electron transfer in syntrophic communities of anaerobic bacteria and archaea.Nat Rev Microbiol. 2009 Aug;7(8):568-77. doi: 10.1038/nrmicro2166. Nat Rev Microbiol. 2009. PMID: 19609258 Review.
Cited by
-
Physicochemical profiles of mixed ruminal microbes in response to surface tension and specific surface area.Front Vet Sci. 2025 Jan 6;11:1514952. doi: 10.3389/fvets.2024.1514952. eCollection 2024. Front Vet Sci. 2025. PMID: 39834927 Free PMC article.
-
Disruption-induced changes in syntrophic propionate and acetate oxidation: flocculation, cell proximity, and microbial activity.Biotechnol Biofuels Bioprod. 2025 Apr 19;18(1):45. doi: 10.1186/s13068-025-02644-3. Biotechnol Biofuels Bioprod. 2025. PMID: 40253350 Free PMC article.
References
-
- Afonso AC, Gomes IB, Saavedra MJ, Giaouris E, Simões LC, Simões M (2021) Bacterial coaggregation in aquatic systems. Water Res 196:117037. 10.1016/j.watres.2021.117037 - PubMed
-
- Aqeel H, Weissbrodt DG, Cerruti M, Wolfaardt GM, Wilén B-M, Liss SN (2019) Drivers of bioaggregation from flocs to biofilms and granular sludge. Environ Sci (camb) 5:2072–2089. 10.1039/C9EW00450E
-
- Belas R (2014) Biofilms, flagella, and mechanosensing of surfaces by bacteria. Trends Microbiol 22:517–527. 10.1016/j.tim.2014.05.002 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

