Nanofeatured surfaces in dental implants: contemporary insights and impending challenges
- PMID: 38963524
- PMCID: PMC11224214
- DOI: 10.1186/s40729-024-00550-1
Nanofeatured surfaces in dental implants: contemporary insights and impending challenges
Abstract
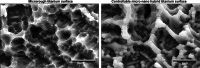
Dental implant therapy, established as standard-of-care nearly three decades ago with the advent of microrough titanium surfaces, revolutionized clinical outcomes through enhanced osseointegration. However, despite this pivotal advancement, challenges persist, including prolonged healing times, restricted clinical indications, plateauing success rates, and a notable incidence of peri-implantitis. This review explores the biological merits and constraints of microrough surfaces and evaluates the current landscape of nanofeatured dental implant surfaces, aiming to illuminate strategies for addressing existing impediments in implant therapy. Currently available nanofeatured dental implants incorporated nano-structures onto their predecessor microrough surfaces. While nanofeature integration into microrough surfaces demonstrates potential for enhancing early-stage osseointegration, it falls short of surpassing its predecessors in terms of osseointegration capacity. This discrepancy may be attributed, in part, to the inherent "dichotomy kinetics" of osteoblasts, wherein increased surface roughness by nanofeatures enhances osteoblast differentiation but concomitantly impedes cell attachment and proliferation. We also showcase a controllable, hybrid micro-nano titanium model surface and contrast it with commercially-available nanofeatured surfaces. Unlike the commercial nanofeatured surfaces, the controllable micro-nano hybrid surface exhibits superior potential for enhancing both cell differentiation and proliferation. Hence, present nanofeatured dental implants represent an evolutionary step from conventional microrough implants, yet they presently lack transformative capacity to surmount existing limitations. Further research and development endeavors are imperative to devise optimized surfaces rooted in fundamental science, thereby propelling technological progress in the field.
Keywords: Bone-titanium integration; Dental and orthopedic implants; Microrough surface; Osseointegration; Osteoblasts.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
A Newly Created Meso-, Micro-, and Nano-Scale Rough Titanium Surface Promotes Bone-Implant Integration.Int J Mol Sci. 2020 Jan 25;21(3):783. doi: 10.3390/ijms21030783. Int J Mol Sci. 2020. PMID: 31991761 Free PMC article.
-
[The role of surface roughness in promoting osteointegration].Refuat Hapeh Vehashinayim (1993). 2003 Jul;20(3):8-19, 98. Refuat Hapeh Vehashinayim (1993). 2003. PMID: 14515625 Review. Hebrew.
-
Beyond microroughness: novel approaches to navigate osteoblast activity on implant surfaces.Int J Implant Dent. 2024 Jul 5;10(1):35. doi: 10.1186/s40729-024-00554-x. Int J Implant Dent. 2024. PMID: 38967690 Free PMC article. Review.
-
Effect of photofunctionalization on fluoride-treated nanofeatured titanium.J Biomater Appl. 2014 Apr;28(8):1200-12. doi: 10.1177/0885328213501566. Epub 2013 Aug 28. J Biomater Appl. 2014. PMID: 23985537
-
Superposition of nanostructures on microrough titanium-aluminum-vanadium alloy surfaces results in an altered integrin expression profile in osteoblasts.Connect Tissue Res. 2014 Aug;55 Suppl 1(0 1):164-8. doi: 10.3109/03008207.2014.923881. Connect Tissue Res. 2014. PMID: 25158204 Free PMC article.
Cited by
-
Nanorough Surface of Fibronectin Grafted Bioactive Zirconia Dental Implants by Using Glow Discharge Plasma Promotes Osseointegration in a Rabbit Model.Int J Nanomedicine. 2024 Nov 25;19:12615-12631. doi: 10.2147/IJN.S494580. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39619054 Free PMC article.
-
The next frontier in osseointegration: energy and speed as critical determinants and their enhancement by UV photofunctionalization.Int J Implant Dent. 2025 Aug 4;11(1):50. doi: 10.1186/s40729-025-00638-2. Int J Implant Dent. 2025. PMID: 40759808 Free PMC article.
-
Designing Superhydrophilic 3D Porous Surfaces on Polyetherketoneketone Surfaces to Promote Biocompatibility.J Funct Biomater. 2025 Mar 14;16(3):106. doi: 10.3390/jfb16030106. J Funct Biomater. 2025. PMID: 40137385 Free PMC article.
-
In Vivo Investigation on the Effects of Metal Oxides in Hydroxyapatite Biocomposite Implants for Improving Bone Regeneration.Biol Trace Elem Res. 2025 May 16. doi: 10.1007/s12011-025-04651-9. Online ahead of print. Biol Trace Elem Res. 2025. PMID: 40380028
-
The 3D theory of osseointegration: material, topography, and time as interdependent determinants of bone-implant integration.Int J Implant Dent. 2025 Aug 2;11(1):49. doi: 10.1186/s40729-025-00639-1. Int J Implant Dent. 2025. PMID: 40751787 Free PMC article. Review.
References
-
- Brånemark PI, Adell R, Breine U, Hansson BO, Lindström J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3(2):81–100. - PubMed
-
- Kitajima H, Hirota M, Iwai T, Mitsudo K, Saruta J, Ogawa T. Synergistic Enhancement of Protein Recruitment and Retention via Implant Surface Microtopography and Superhydrophilicity in a computational Fluid Dynamics Model. Int J Mol Sci. 2023;24:15618. doi: 10.3390/ijms242115618. - DOI - PMC - PubMed

