Preparation of poly(vinyl alcohol) nanofibers containing disulfiram-copper complex by electrospinning: a potential delivery system against melanoma
- PMID: 38963538
- PMCID: PMC11554976
- DOI: 10.1007/s40199-024-00527-w
Preparation of poly(vinyl alcohol) nanofibers containing disulfiram-copper complex by electrospinning: a potential delivery system against melanoma
Abstract
Background: Melanoma poses a significant threat to human health, making the development of a safe and effective treatment a crucial challenge. Disulfiram (DS) is a proven anticancer drug that has shown effectiveness when used in combination with copper (DS-Cu complex).
Objectives: This study focuses on encapsulation of DS-copper complex into nanofiber scaffold from polyvinyl alcohol (PVA) (DS-Cu@PVA). In order to increase bioavailability towards melanoma cell lines and decrease its toxicity.
Methods: The scaffold was fabricated through an electrospinning process using an aqueous solution, and subsequently analyzed using ART-Fourier transform infrared spectroscopy (ART-FTIR), scanning electron microscopy (SEM), and energy dispersive X-ray analysis (EDX). Additionally, cellular cytotoxicity, flow cytometry analysis, and determination of caspase 3 activity were conducted to further characterize the scaffold.
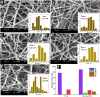
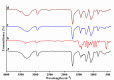
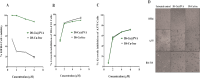
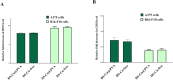
Results: The results confirmed that encapsulation of DS-Cu complex into PVA was successful via different characterization. The scanning electron microscopy (SEM) analysis revealed that the diameter of the nanofibers remained consistent despite the addition of DS-Cu. Additionally, ATR-FTIR confirmed that the incorporation of DS-Cu into PVA did not significantly alter the characteristic peaks of PVA. Furthermore, the cytotoxicity assessment of the DS-Cu@PVA nanofibrous scaffold using human normal skin cells (HFB4) demonstrated its superior biocompatibility compared to DS-Cu-free counterparts. Notably, the presence of DS-Cu maintained its effectiveness in promoting apoptosis by increasing cellular reactive oxygen species, proapoptotic gene expression, and caspase 3 activity, while simultaneously reducing glutathione levels and oncogene expression in human and mouse melanoma cell lines (A375 and B16F10, respectively). Overall, these findings suggest that the addition of DS-Cu to PVA nanofibers enhances their biocompatibility and cytotoxic effects on melanoma cells, making them a promising candidate for biomedical applications.
Conclusion: The findings indicate that the targeted delivery of DS-Cu onto a PVA nanofiber scaffold holds potential approach to enhance the efficacy of DS-Cu in combating melanoma.
Keywords: Anticancer; Disulfiram; Electrospinning; Melanoma; Polyvinyl alcohol.
© 2024. The Author(s), under exclusive licence to Tehran University of Medical Sciences.
Conflict of interest statement
Figures

Similar articles
-
Quality-by-design-based microemulsion of disulfiram for repurposing in melanoma and breast cancer therapy.Ther Deliv. 2024;15(7):521-544. doi: 10.1080/20415990.2024.2363136. Epub 2024 Jul 1. Ther Deliv. 2024. PMID: 38949622 Free PMC article.
-
Core-shell nanofibers for localized melanoma therapy delivering Pioglitazone nanoemulsions and gemcitabine dual loaded system.Sci Rep. 2025 Aug 4;15(1):28401. doi: 10.1038/s41598-025-14483-1. Sci Rep. 2025. PMID: 40760166 Free PMC article.
-
Exploiting mitochondrial dysfunction to overcome BRAF inhibitor resistance in advanced melanoma: the role of disulfiram as a copper ionophore.Cell Death Dis. 2025 Jul 1;16(1):482. doi: 10.1038/s41419-025-07766-y. Cell Death Dis. 2025. PMID: 40592836 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
Cited by
-
Reverse Gradient Distributions of Drug and Polymer Molecules within Electrospun Core-Shell Nanofibers for Sustained Release.Int J Mol Sci. 2024 Sep 1;25(17):9524. doi: 10.3390/ijms25179524. Int J Mol Sci. 2024. PMID: 39273471 Free PMC article.
-
Synergistic Effects of Radical Distributions of Soluble and Insoluble Polymers within Electrospun Nanofibers for an Extending Release of Ferulic Acid.Polymers (Basel). 2024 Sep 15;16(18):2614. doi: 10.3390/polym16182614. Polymers (Basel). 2024. PMID: 39339078 Free PMC article.
-
Copper in melanoma: At the crossroad of protumorigenic and anticancer roles.Redox Biol. 2025 Apr;81:103552. doi: 10.1016/j.redox.2025.103552. Epub 2025 Feb 15. Redox Biol. 2025. PMID: 39970778 Free PMC article. Review.
References
-
- Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Robert S, et al. Development and validation of a novel model to predict recurrence-free survival and melanoma-specific survival after sentinel lymph node biopsy in patients with melanoma: an international, retrospective, multicentre analysis. Lancet Oncol. 2024;4:509–17. - PubMed
-
- Jiaran Z, Huichun T, Lili M, Lu S. Treatment of acral and mucosal melanoma: current and emerging targeted therapies. Crit Rev Oncol Hematol. 2024;193:104221. - PubMed
-
- Ruilong W, Qin Y, Xiao L, Jinfeng W. Unraveling lipid metabolism reprogramming for overcoming drug resistance in melanoma. Biochem Pharmacol. 2024;223:116122. - PubMed
-
- Cen D, et al. Disulfiram induces apoptosis in human melanoma cells: a redox-related process. Mol Cancer Ther. 2002;1:197–204. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

