A dynamic flow fetal membrane organ-on-a-chip system for modeling the effects of amniotic fluid motion
- PMID: 38963644
- PMCID: PMC11624963
- DOI: 10.1007/s10544-024-00714-1
A dynamic flow fetal membrane organ-on-a-chip system for modeling the effects of amniotic fluid motion
Abstract
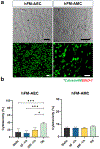
Fetal membrane (amniochorion), the innermost lining of the intrauterine cavity, surround the fetus and enclose amniotic fluid. Unlike unidirectional blood flow, amniotic fluid subtly rocks back and forth, and thus, the innermost amnion epithelial cells are continuously exposed to low levels of shear stress from fluid undulation. Here, we tested the impact of fluid motion on amnion epithelial cells (AECs) as a bearer of force impact and their potential vulnerability to cytopathologic changes that can destabilize fetal membrane functions. A previously developed amnion membrane (AM) organ-on-chip (OOC) was utilized but with dynamic flow to culture human fetal amnion membrane cells. The applied flow was modulated to perfuse culture media back and forth for 48 h to mimic fluid motion. A static culture condition was used as a negative control, and oxidative stress (OS) condition was used as a positive control representing pathophysiological changes. The impacts of fluidic motion were evaluated by measuring cell viability, cellular transition, and inflammation. Additionally, scanning electron microscopy (SEM) imaging was performed to observe microvilli formation. The results show that regardless of the applied flow rate, AECs and AMCs maintained their viability, morphology, innate meta-state, and low production of pro-inflammatory cytokines. E-cadherin expression and microvilli formation in the AECs were upregulated in a flow rate-dependent fashion; however, this did not impact cellular morphology or cellular transition or inflammation. OS treatment induced a mesenchymal morphology, significantly higher vimentin to cytokeratin 18 (CK-18) ratio, and pro-inflammatory cytokine production in AECs, whereas AMCs did not respond in any significant manner. Fluid motion and shear stress, if any, did not impact AEC cell function and did not cause inflammation. Thus, when using an amnion membrane OOC model, the inclusion of a dynamic flow environment is not necessary to mimic in utero physiologic cellular conditions of an amnion membrane.
Keywords: Amniotic fluid; Dynamic flow cell culture; Fetal membrane; Microphysiological system; Organ-on-chip; Preterm birth; Shear stress.
© 2024. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures
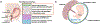



Update of
-
A Dynamic Flow Fetal Membrane Organ-on-a-Chip System for Modeling the Effects of Amniotic Fluid Motion.Res Sq [Preprint]. 2024 May 15:rs.3.rs-4372328. doi: 10.21203/rs.3.rs-4372328/v1. Res Sq. 2024. Update in: Biomed Microdevices. 2024 Jul 4;26(3):32. doi: 10.1007/s10544-024-00714-1. PMID: 38798515 Free PMC article. Updated. Preprint.
Similar articles
-
A Dynamic Flow Fetal Membrane Organ-on-a-Chip System for Modeling the Effects of Amniotic Fluid Motion.Res Sq [Preprint]. 2024 May 15:rs.3.rs-4372328. doi: 10.21203/rs.3.rs-4372328/v1. Res Sq. 2024. Update in: Biomed Microdevices. 2024 Jul 4;26(3):32. doi: 10.1007/s10544-024-00714-1. PMID: 38798515 Free PMC article. Updated. Preprint.
-
Amnioinfusion for chorioamnionitis.Cochrane Database Syst Rev. 2016 Aug 24;2016(8):CD011622. doi: 10.1002/14651858.CD011622.pub2. Cochrane Database Syst Rev. 2016. PMID: 27556818 Free PMC article.
-
Conjunctival autograft for pterygium.Cochrane Database Syst Rev. 2016 Feb 11;2(2):CD011349. doi: 10.1002/14651858.CD011349.pub2. Cochrane Database Syst Rev. 2016. PMID: 26867004 Free PMC article.
-
Novel application of metabolic imaging of early embryos using a light-sheet on-a-chip device: a proof-of-concept study.Hum Reprod. 2025 Jan 1;40(1):41-55. doi: 10.1093/humrep/deae249. Hum Reprod. 2025. PMID: 39521726 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Physical model of serum supplemented medium flow in organ-on-a-chip systems.PLoS One. 2025 Jun 17;20(6):e0322069. doi: 10.1371/journal.pone.0322069. eCollection 2025. PLoS One. 2025. PMID: 40526698 Free PMC article.
-
Amniotic membrane, a novel bioscaffold in cardiac diseases: from mechanism to applications.Front Bioeng Biotechnol. 2024 Dec 20;12:1521462. doi: 10.3389/fbioe.2024.1521462. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39758951 Free PMC article. Review.
-
Modeling reproductive and pregnancy-associated tissues using organ-on-chip platforms: challenges, limitations, and the high throughput data frontier.Front Bioeng Biotechnol. 2025 Apr 1;13:1568389. doi: 10.3389/fbioe.2025.1568389. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40236940 Free PMC article. Review.
References
-
- (1970). "Amniotic fluid: its physiology and pathology." J Reprod Med 5(6): 221–241. - PubMed
-
- Ballermann BJ, Dardik A, Eng E and Liu A (1998). "Shear stress and the endothelium." Kidney Int Suppl 67: S100–108. - PubMed
-
- Borgida AF, Mills AA, Feldman DM, Rodis JF and Egan JF (2000). "Outcome of pregnancies complicated by ruptured membranes after genetic amniocentesis." Am J Obstet Gynecol 183(4): 937–939. - PubMed
MeSH terms
Grants and funding
- R01 HD100729/HD/NICHD NIH HHS/United States
- UH2 TR004117/TR/NCATS NIH HHS/United States
- UH3 TR003283/TR/NCATS NIH HHS/United States
- 1R01HD100729/National Institute of Child Health and Human Development
- UG3 TR003283/TR/NCATS NIH HHS/United States
- P42 ES027704/ES/NIEHS NIH HHS/United States
- UH3 TR004117/TR/NCATS NIH HHS/United States
- R01 HD110400/HD/NICHD NIH HHS/United States
- UG3TR003283/UH3TR003283/TR/NCATS NIH HHS/United States
- R01HD110400/National Institute of Child Health and Human Development
- P42ES027704/ES/NIEHS NIH HHS/United States
- U2C TR004868/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

