CB2 stimulation of adipose resident ILC2s orchestrates immune balance and ameliorates type 2 diabetes mellitus
- PMID: 38963763
- PMCID: PMC11317174
- DOI: 10.1016/j.celrep.2024.114434
CB2 stimulation of adipose resident ILC2s orchestrates immune balance and ameliorates type 2 diabetes mellitus
Abstract
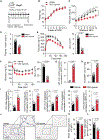
Development of type 2 diabetes mellitus (T2DM) is associated with low-grade chronic type 2 inflammation and disturbance of glucose homeostasis. Group 2 innate lymphoid cells (ILC2s) play a critical role in maintaining adipose homeostasis via the production of type 2 cytokines. Here, we demonstrate that CB2, a G-protein-coupled receptor (GPCR) and member of the endocannabinoid system, is expressed on both visceral adipose tissue (VAT)-derived murine and human ILC2s. Moreover, we utilize a combination of ex vivo and in vivo approaches to explore the functional and therapeutic impacts of CB2 engagement on VAT ILC2s in a T2DM model. Our results show that CB2 stimulation of ILC2s protects against insulin-resistance onset, ameliorates glucose tolerance, and reverses established insulin resistance. Our mechanistic studies reveal that the therapeutic effects of CB2 are mediated through activation of the AKT, ERK1/2, and CREB pathways on ILC2s. The results reveal that the CB2 agonist can serve as a candidate for the prevention and treatment of T2DM.
Keywords: CB2; CP: Immunology; CP: Metabolism; ILC2; T2DM; adipose inflammation; glucose tolerance; immunotherapy; insulin resistance.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





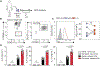
Similar articles
-
DR3 stimulation of adipose resident ILC2s ameliorates type 2 diabetes mellitus.Nat Commun. 2020 Sep 18;11(1):4718. doi: 10.1038/s41467-020-18601-7. Nat Commun. 2020. PMID: 32948777 Free PMC article.
-
Costimulation of type-2 innate lymphoid cells by GITR promotes effector function and ameliorates type 2 diabetes.Nat Commun. 2019 Feb 12;10(1):713. doi: 10.1038/s41467-019-08449-x. Nat Commun. 2019. PMID: 30755607 Free PMC article.
-
ILC2s Improve Glucose Metabolism Through the Control of Saturated Fatty Acid Absorption Within Visceral Fat.Front Immunol. 2021 Jul 9;12:669629. doi: 10.3389/fimmu.2021.669629. eCollection 2021. Front Immunol. 2021. PMID: 34305899 Free PMC article.
-
Type 2 Innate Lymphoid Cells: Protectors in Type 2 Diabetes.Front Immunol. 2021 Aug 19;12:727008. doi: 10.3389/fimmu.2021.727008. eCollection 2021. Front Immunol. 2021. PMID: 34489979 Free PMC article. Review.
-
Therapeutic potential of cannabinoid receptor 2 in the treatment of diabetes mellitus and its complications.Eur J Pharmacol. 2019 Nov 5;862:172628. doi: 10.1016/j.ejphar.2019.172628. Epub 2019 Aug 25. Eur J Pharmacol. 2019. PMID: 31461639 Review.
Cited by
-
The indispensability of methyltransferase-like 3 in the immune system: from maintaining homeostasis to driving function.Front Immunol. 2024 Oct 2;15:1456891. doi: 10.3389/fimmu.2024.1456891. eCollection 2024. Front Immunol. 2024. PMID: 39416774 Free PMC article. Review.
-
FOXO1 pathway activation by VISTA immune checkpoint restrains pulmonary ILC2 functions.J Clin Invest. 2025 Jan 2;135(4):e184932. doi: 10.1172/JCI184932. J Clin Invest. 2025. PMID: 39745792 Free PMC article.
-
Targeting the Endocannabinoidome: A Novel Approach to Managing Extraintestinal Complications in Inflammatory Bowel Disease.Pharmaceuticals (Basel). 2025 Mar 27;18(4):478. doi: 10.3390/ph18040478. Pharmaceuticals (Basel). 2025. PMID: 40283915 Free PMC article. Review.
-
BTLA agonist attenuates Th17-driven inflammation in a mouse model of steroid-resistant asthma.Front Immunol. 2025 Mar 28;16:1552394. doi: 10.3389/fimmu.2025.1552394. eCollection 2025. Front Immunol. 2025. PMID: 40226621 Free PMC article.
References
-
- Hurrell BP, Sakano Y, Shen S, Helou DG, Li M, Shafiei-Jahani P, Kazemi MH, Sakano K, Li X, Quach C, et al. (2024). Iron controls the development of airway hyperreactivity by regulating ILC2 metabolism and effector function. Sci. Transl. Med. 16, eadk4728. 10.1126/scitranslmed.adk4728. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

