A pH-sensitive closed-loop nanomachine to control hyperexcitability at the single neuron level
- PMID: 38965228
- PMCID: PMC11224301
- DOI: 10.1038/s41467-024-49941-3
A pH-sensitive closed-loop nanomachine to control hyperexcitability at the single neuron level
Abstract
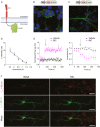
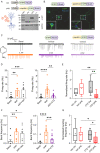
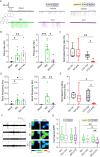
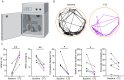
Epilepsy affects 1% of the general population and 30% of patients are resistant to antiepileptic drugs. Although optogenetics is an efficient antiepileptic strategy, the difficulty of illuminating deep brain areas poses translational challenges. Thus, the search of alternative light sources is strongly needed. Here, we develop pH-sensitive inhibitory luminopsin (pHIL), a closed-loop chemo-optogenetic nanomachine composed of a luciferase-based light generator, a fluorescent sensor of intracellular pH (E2GFP), and an optogenetic actuator (halorhodopsin) for silencing neuronal activity. Stimulated by coelenterazine, pHIL experiences bioluminescence resonance energy transfer between luciferase and E2GFP which, under conditions of acidic pH, activates halorhodopsin. In primary neurons, pHIL senses the intracellular pH drop associated with hyperactivity and optogenetically aborts paroxysmal activity elicited by the administration of convulsants. The expression of pHIL in hippocampal pyramidal neurons is effective in decreasing duration and increasing latency of pilocarpine-induced tonic-clonic seizures upon in vivo coelenterazine administration, without affecting higher brain functions. The same treatment is effective in markedly decreasing seizure manifestations in a murine model of genetic epilepsy. The results indicate that pHIL represents a potentially promising closed-loop chemo-optogenetic strategy to treat drug-refractory epilepsy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

