Cancer stem cells: advances in knowledge and implications for cancer therapy
- PMID: 38965243
- PMCID: PMC11224386
- DOI: 10.1038/s41392-024-01851-y
Cancer stem cells: advances in knowledge and implications for cancer therapy
Abstract
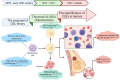
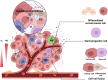
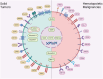
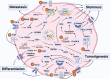
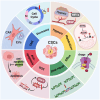
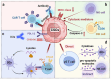
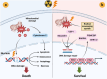
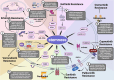
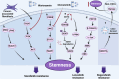
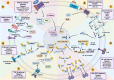
Cancer stem cells (CSCs), a small subset of cells in tumors that are characterized by self-renewal and continuous proliferation, lead to tumorigenesis, metastasis, and maintain tumor heterogeneity. Cancer continues to be a significant global disease burden. In the past, surgery, radiotherapy, and chemotherapy were the main cancer treatments. The technology of cancer treatments continues to develop and advance, and the emergence of targeted therapy, and immunotherapy provides more options for patients to a certain extent. However, the limitations of efficacy and treatment resistance are still inevitable. Our review begins with a brief introduction of the historical discoveries, original hypotheses, and pathways that regulate CSCs, such as WNT/β-Catenin, hedgehog, Notch, NF-κB, JAK/STAT, TGF-β, PI3K/AKT, PPAR pathway, and their crosstalk. We focus on the role of CSCs in various therapeutic outcomes and resistance, including how the treatments affect the content of CSCs and the alteration of related molecules, CSCs-mediated therapeutic resistance, and the clinical value of targeting CSCs in patients with refractory, progressed or advanced tumors. In summary, CSCs affect therapeutic efficacy, and the treatment method of targeting CSCs is still difficult to determine. Clarifying regulatory mechanisms and targeting biomarkers of CSCs is currently the mainstream idea.
© 2024. The Author(s).
Conflict of interest statement
The author declares no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

