A solid state electrolyte based enzymatic acetone sensor
- PMID: 38965300
- PMCID: PMC11224388
- DOI: 10.1038/s41598-024-66498-9
A solid state electrolyte based enzymatic acetone sensor
Abstract
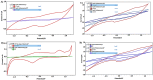
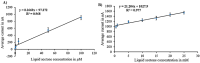
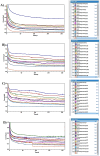
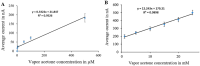
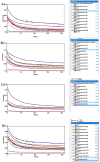
This paper introduces a novel solid-state electrolyte-based enzymatic sensor designed for the detection of acetone, along with an examination of its performance under various surface modifications aimed at optimizing its sensing capabilities. To measure acetone concentrations in both liquid and vapor states, cyclic voltammetry and amperometry techniques were employed, utilizing disposable screen-printed electrodes consisting of a platinum working electrode, a platinum counter electrode, and a silver reference electrode. Four different surface modifications, involving different combinations of Nafion (N) and enzyme (E) layers (N + E; N + E + N; N + N + E; N + N + E + N), were tested to identify the most effective configuration for a sensor that can be used for breath acetone detection. The sensor's essential characteristics, including linearity, sensitivity, reproducibility, and limit of detection, were thoroughly evaluated through a range of experiments spanning concentrations from 1 µM to 25 mM. Changes in acetone concentration were monitored by comparing currents readings at different acetone concentrations. The sensor exhibited high sensitivity, and a linear response to acetone concentration in both liquid and gas phases within the specified concentration range, with correlation coefficients ranging from 0.92 to 0.98. Furthermore, the sensor achieved a rapid response time of 30-50 s and an impressive detection limit as low as 0.03 µM. The results indicated that the sensor exhibited the best linearity, sensitivity, and limit of detection when four layers were employed (N + N + E + N).
Keywords: Acetone; Amperometry; Cyclic voltammetry; Electrochemistry; Enzymatic; Nafion; Sensor.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

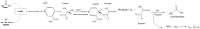







References
-
- Weber I, Derron N, Koenigstein K, Gerber P, Güntner A, Pratsinis S. Monitoring lipolysis by sensing breath acetone down to ppb. Small Sci. 2021 doi: 10.1002/smsc.202100004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

