Asymptomatic herpes simplex virus brain infection elicits cellular senescence phenotypes in the central nervous system of mice suffering multiple sclerosis-like disease
- PMID: 38965360
- PMCID: PMC11224417
- DOI: 10.1038/s42003-024-06486-x
Asymptomatic herpes simplex virus brain infection elicits cellular senescence phenotypes in the central nervous system of mice suffering multiple sclerosis-like disease
Abstract
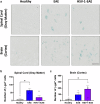
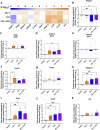
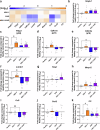
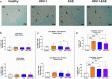
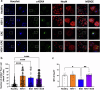
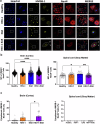
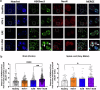
Experimental autoimmune encephalomyelitis (EAE) is a demyelinating disease affecting the central nervous system (CNS) in animals that parallels several clinical and molecular traits of multiple sclerosis in humans. Herpes simplex virus type 1 (HSV-1) infection mainly causes cold sores and eye diseases, yet eventually, it can also reach the CNS, leading to acute encephalitis. Notably, a significant proportion of healthy individuals are likely to have asymptomatic HSV-1 brain infection with chronic brain inflammation due to persistent latent infection in neurons. Because cellular senescence is suggested as a potential factor contributing to the development of various neurodegenerative disorders, including multiple sclerosis, and viral infections may induce a premature senescence state in the CNS, potentially increasing susceptibility to such disorders, here we examine the presence of senescence-related markers in the brains and spinal cords of mice with asymptomatic HSV-1 brain infection, EAE, and both conditions. Across all scenarios, we find a significant increases of senescence biomarkers in the CNS with some differences depending on the analyzed group. Notably, some senescence biomarkers are exclusively observed in mice with the combined conditions. These results indicate that asymptomatic HSV-1 brain infection and EAE associate with a significant expression of senescence biomarkers in the CNS.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Thomas, F. P. in Pathy’s Principles and Practice of Geriatric Medicine. (Sinclair, A. J., Vellas, B. & Morley, J. E. eds) Ch. 70 (Wiley, 2012).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

