Rearrangements involving 11q23.3/KMT2A in adult AML: mutational landscape and prognostic implications - a HARMONY study
- PMID: 38965370
- PMCID: PMC11347382
- DOI: 10.1038/s41375-024-02333-4
Rearrangements involving 11q23.3/KMT2A in adult AML: mutational landscape and prognostic implications - a HARMONY study
Abstract
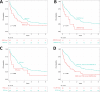
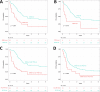
Balanced rearrangements involving the KMT2A gene (KMT2Ar) are recurrent genetic abnormalities in acute myeloid leukemia (AML), but there is lack of consensus regarding the prognostic impact of different fusion partners. Moreover, prognostic implications of gene mutations co-occurring with KMT2Ar are not established. From the HARMONY AML database 205 KMT2Ar adult patients were selected, 185 of whom had mutational information by a panel-based next-generation sequencing analysis. Overall survival (OS) was similar across the different translocations, including t(9;11)(p21.3;q23.3)/KMT2A::MLLT3 (p = 0.756). However, independent prognostic factors for OS in intensively treated patients were age >60 years (HR 2.1, p = 0.001), secondary AML (HR 2.2, p = 0.043), DNMT3A-mut (HR 2.1, p = 0.047) and KRAS-mut (HR 2.0, p = 0.005). In the subset of patients with de novo AML < 60 years, KRAS and TP53 were the prognostically most relevant mutated genes, as patients with a mutation of any of those two genes had a lower complete remission rate (50% vs 86%, p < 0.001) and inferior OS (median 7 vs 30 months, p < 0.001). Allogeneic hematopoietic stem cell transplantation in first complete remission was able to improve OS (p = 0.003). Our study highlights the importance of the mutational patterns in adult KMT2Ar AML and provides new insights into more accurate prognostic stratification of these patients.
© 2024. The Author(s).
Conflict of interest statement
MS: honoraria from Novartis, Celgene, AOP Orphan, AbbVie. KHM: honoraria from AbbVie, Bristol Myers Squibb, Celgene, Janssen, Novartis, Pfizer, Otsuka; research funding from AbbVie. RA: honoraria from Astellas, Bristol Myers Squibb, Incyte, and Novartis. MP: honoraria from Novartis. CT: co-owner and CEO of AgenDix GmbH and has received lecture fees and/or participated in Ad-Boards from Novartis, Jazz Pharmaceuticals, Astellas, Janssen, Illumina; research funding from Novartis, Bayer. GS: honoraria from Takeda and has participated in Ad-Boards from Novartis, Celgene, Abbvie, Helsinn and Takeda. KD: honoraria from Novartis, Jazz Pharmaceuticals, Abbvie; has participated in Ad-Boards from Novartis, Bristol Myers Squibb, Jazz Pharmaceuticals, Abbvie; research funding from Novartis, Astellas, Agios, Bristol Myers Squibb, Kronos. MH: honoraria from Takeda, Novartis, Janssen, Jazz Pharmaceuticals, Eurocept, Abbvie and has participated in Ad-Boards from Kura Oncology, Glycostem, Daiichi Sankyo, Bristol Myers Squibb, Novartis, Jazz Pharmaceuticals, Abbvie, Pfizer, PinotBio, Roche, Tolremo; research funding from Glycostem, Daiichi Sankyo, Bristol Myers Squibb, Novartis, Jazz Pharmaceuticals, Abbvie, Pfizer, PinotBio, Roche, Astellas, Bayer, BergenBio, Loxo Oncology. TH: current employment at Munich Leukemia Laboratory, with part ownership. ATT: Consultancy for CSL Behring, Maat Pharma, Biomarin and Onkowissen; travel reimbursements from Neovii Biotech. DR: has participated in Ad-Boards from Bristol Myers Squibb, Novartis, Cerus, Medac, EUSA Pharma, Bluebird Bio; research funding from Novartis, BlueBird Bio. RSR: current employment at Bayer Pharma AG. MB: current employment at Abbvie. JMHR: honoraria from Bristol Myers Squibb, Pfizer, Amgen, Celgene, GSK, Novartis; advisory role for Bristol Myers Squibb, Pfizer, Amgen, Celgene, Novartis, Janssen, Roche, Abbvie, AstraZeneca, Beigene, Lilly, Gilead, Takeda, Jazz Pharmaceuticals, Rovi, Incyte; research funding from Bristol Myers Squibb, Celgene, Novartis. BH: honoraria from Pfizer, Bristol Myers Squibb, Novartis; research funding from AstraZeneca. GO: honoraria from Abbvie, Jazz Pharmaceuticals, Astellas, Gilead, Bristol Myers Squibb, Servier, Roche. HD: advisory role for AbbVie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, Bristol Myers Squibb, Celgene, GEMoaB, Gilead Sciences, Janssen, Jazz Pharmaceuticals, Novartis, Syndax; research funding from AbbVie, Agios, Amgen, Astellas, Bristol Myers Squibb, Jazz Pharmaceuticals, Kronos-Bio, Novartis. LB: honoraria from AbbVie, Amgen, Astellas, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Hexal, Janssen, Jazz Pharmaceuticals, Menarini, Novartis, Pfizer, Roche and Sanofi; research funding from Bayer, Jazz Pharmaceuticals.
Figures



References
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

