Direct observation of translational activation by a ribonucleoprotein granule
- PMID: 38965420
- PMCID: PMC11321996
- DOI: 10.1038/s41556-024-01452-5
Direct observation of translational activation by a ribonucleoprotein granule
Abstract
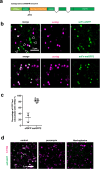
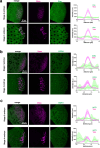
Biomolecular condensates organize biochemical processes at the subcellular level and can provide spatiotemporal regulation within a cell. Among these, ribonucleoprotein (RNP) granules are storage hubs for translationally repressed mRNA. Whether RNP granules can also activate translation and how this could be achieved remains unclear. Here, using single-molecule imaging, we demonstrate that the germ cell-determining RNP granules in Drosophila embryos are sites for active translation of nanos mRNA. Nanos translation occurs preferentially at the germ granule surface with the 3' UTR buried within the granule. Smaug, a cytosolic RNA-binding protein, represses nanos translation, which is relieved when Smaug is sequestered to the germ granule by the scaffold protein Oskar. Together, our findings uncover a molecular process by which RNP granules achieve localized protein synthesis through the compartmentalized loss of translational repression.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

