Persistent effectiveness of CGRP antibody therapy in migraine and comorbid medication overuse or medication overuse headache - a retrospective real-world analysis
- PMID: 38965463
- PMCID: PMC11225246
- DOI: 10.1186/s10194-024-01813-3
Persistent effectiveness of CGRP antibody therapy in migraine and comorbid medication overuse or medication overuse headache - a retrospective real-world analysis
Abstract
Background: Management of patients with migraine who have concomitant medication overuse (MO) or medication overuse headache (MOH) is a major problem in clinical practice. Detoxification of acute analgesics before or during initiation of prophylactic therapy has long been recommended although this concept has recently been questioned. Additionally, relapse after detoxification is a common problem. This real-world study analyses the initial and sustained effectiveness of prophylactic migraine therapy with CGRP (receptor) antibodies without prior detoxification in patients with comorbid MO or MOH for up to one year.
Methods: A retrospective real-world analysis was performed on 291 patients (episodic migraine (EM) with MO (EM-MO; n = 35), EM without MO (EM-noMO; n = 77), chronic migraine (CM) with MOH (CM-MOH; n = 109), CM without MOH (CM-noMOH; n = 70). All patients began treatment with either erenumab (n = 173), fremanezumab (n = 70) or galcanezumab (n = 48) without prior detoxification. Data were available for up to 12 months of treatment. Responder rates for monthly headache days (MHD), monthly migraine days (MMD) and monthly acute medication intake (AMD) were analysed.
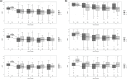
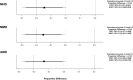
Results: All groups showed a significant reduction in MHD, MMD and AMD at the last observed time point compared to baseline. In patients with CM and MOH, 60.6% (66/109) no longer fulfilled the definition of MO or MOH and a further 13.8% (15/109) had only EM-MO. In the EM cohort, 89% (31/35) of MO patients lost their MO during therapy. MHD and AMD 30% responder rates were comparable for CM-MOH and CM-noMOH (MHD: CM-MOH: 56.0% vs. CM-noMOH: 41.4%, p = 0.058, AMD: CM-MOH: 66.1% vs. CM-noMOH: 52.9%, p = 0.077). MMD responder rate did not differ significantly (after Bonferroni adjustment) (CM-MOH: 62.4% vs. CM-noMOH: 47.1%, p = 0.045, α = 0.017). After successful initiation of therapy, 15.4% of the initial CM-MOH patients relapsed and met the criterion for CM-MOH at the end of follow-up. There were no antibody specific differences in response to therapy.
Conclusions: Our data confirms the effectiveness of CGRP antibody treatment in migraine patients with additional MOH or MO in a real-world setting. Low relapse rates after initial successful therapy support an early start of CGRP antibody treatment in patients with MOH or MO.
Trial registration: No registration, retrospective analysis.
Keywords: Chronic migraine; Detoxification; Erenumab; Fremanezumab; Galcanezumab; MO; MOH.
© 2024. The Author(s).
Conflict of interest statement
DH has received scientific support and/or honoraria from Biogen, Novartis, Lilly, Sanofi-Aventis, Teva, Allergan, Hormosan.
CK has received honoraria, a consulting or advisory role to declare from Novartis and Teva.
MN received travel fees from Licher MT.
AS has received travel fees from Teva and honoraria from Novartis (advisory board).
JB, WB, DF, VB, DL, LM and HS declare that there is no conflict of interest.
Figures



References
-
- Headache Classification Committee of the International Headache Society (IHS) (2018) The international classification of headache disorders, 3rd edition. Cephalalgia 38:1–211. 10.1177/0333102417738202 - PubMed
-
- Linde M, Gustavsson A, Stovner LJ et al (2012) The cost of headache disorders in Europe: the Eurolight project. Euro J Neurol 19:703–711. 10.1111/j.1468-1331.2011.03612.x - PubMed
-
- Diener H-C, Holle D, Solbach K, Gaul C (2016) Medication-overuse headache: risk factors, pathophysiology and management. Nat Rev Neurol 12:575–583. 10.1038/nrneurol.2016.124 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

