Injective hydrogel loaded with liposomes-encapsulated MY-1 promotes wound healing and increases tensile strength by accelerating fibroblast migration via the PI3K/AKT-Rac1 signaling pathway
- PMID: 38965546
- PMCID: PMC11225333
- DOI: 10.1186/s12951-024-02666-3
Injective hydrogel loaded with liposomes-encapsulated MY-1 promotes wound healing and increases tensile strength by accelerating fibroblast migration via the PI3K/AKT-Rac1 signaling pathway
Abstract
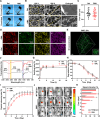
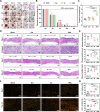
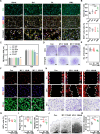
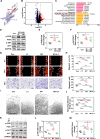
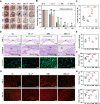
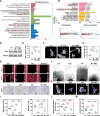
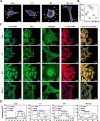
Failed skin wound healing, through delayed wound healing or wound dehiscence, is a global public health issue that imposes significant burdens on individuals and society. Although the application of growth factor is an effective method to improve the pace and quality of wound healing, the clinically approved factors are limited. Parathyroid hormone (PTH) demonstrates promising results in wound healing by promoting collagen deposition and cell migration, but its application is limited by potentially inhibitory effects when administered continuously and locally. Through partially replacing and repeating the amino acid domains of PTH(1-34), we previously designed a novel PTH analog, PTH(3-34)(29-34) or MY-1, and found that it avoided the inhibitory effects of PTH while retaining its positive functions. To evaluate its role in wound healing, MY-1 was encapsulated in liposomes and incorporated into the methacryloyl gelatin (GelMA) hydrogel, through which an injectable nanocomposite hydrogel (GelMA-MY@Lipo, or GML) was developed. In vitro studies revealed that the GML had similar properties in terms of the appearance, microstructure, functional groups, swelling, and degradation capacities as the GelMA hydrogel. In vitro drug release testing showed a relatively more sustainable release of MY-1, which was still detectable in vivo 9 days post-application. When the GML was topically applied to the wound areas of rat models, wound closure as well as tensile strength were improved. Further studies showed that the effects of GML on wound repair and tensile strength were closely related to the promotion of fibroblast migration to the wound area through the controlled release of MY-1. Mechanically, MY-1 enhanced fibroblast migration by activating PI3K/AKT signaling and its downstream molecule, Rac1, by which it increased fibroblast aggregation in the early stage and resulting in denser collagen deposition at a later time. Overall, these findings demonstrated that the nanocomposite hydrogel system promoted skin wound healing and increased tensile strength, thus offering new potential in the treatment of wound healing.
Keywords: Cell migration; GelMA; Liposome; PI3K/AKT; Rac1; Wound healing; hPTH(3–34)(29–34).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Human Parathyroid Hormone Analog (3-34/29-34) promotes wound re-epithelialization through inducing keratinocyte migration and epithelial-mesenchymal transition via PTHR1-PI3K/AKT activation.Cell Commun Signal. 2023 Aug 23;21(1):217. doi: 10.1186/s12964-023-01243-9. Cell Commun Signal. 2023. PMID: 37612710 Free PMC article.
-
GelMA hydrogel-loaded extracellular vesicles derived from keratinocytes promote skin microvasculature regeneration and wound healing in diabetic mice through activation of the PDGF-induced PI3K/AKT pathway.Cell Biol Toxicol. 2025 Jun 14;41(1):103. doi: 10.1007/s10565-025-10062-2. Cell Biol Toxicol. 2025. PMID: 40515797 Free PMC article.
-
Effects of Periploca forrestii Schltr on wound healing by Src meditated Mek/Erk and PI3K/Akt signals.J Ethnopharmacol. 2019 Jun 12;237:116-127. doi: 10.1016/j.jep.2019.03.046. Epub 2019 Mar 21. J Ethnopharmacol. 2019. PMID: 30905787
-
The activation of the NF-κB-JNK pathway is independent of the PI3K-Rac1-JNK pathway involved in the bFGF-regulated human fibroblast cell migration.J Dermatol Sci. 2016 Apr;82(1):28-37. doi: 10.1016/j.jdermsci.2016.01.003. Epub 2016 Jan 7. J Dermatol Sci. 2016. PMID: 26829882
-
The Promoting Effect of Animal Bioactive Proteins and Peptide Components on Wound Healing: A Review.Int J Mol Sci. 2024 Nov 22;25(23):12561. doi: 10.3390/ijms252312561. Int J Mol Sci. 2024. PMID: 39684273 Free PMC article. Review.
Cited by
-
An integrin-based quercetin 7-rhamnoside liver-targeted delivery liposomes for intrahepatic cholestasis in pregnancy.Mater Today Bio. 2025 Jun 27;33:102031. doi: 10.1016/j.mtbio.2025.102031. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40677400 Free PMC article.
-
5'tiRNA-Glu-TTC targets TRPV3 and activates the PI3K/AKT signaling pathway to modulate skin photoaging.Noncoding RNA Res. 2025 Jul 14;15:29-43. doi: 10.1016/j.ncrna.2025.07.004. eCollection 2025 Dec. Noncoding RNA Res. 2025. PMID: 40741358 Free PMC article.
References
-
- Rosen RD. and B M. Wound Dehiscence. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. https://www.ncbi.nlm.nih.gov/books/NBK551712/?report.
MeSH terms
Substances
Grants and funding
- 82271616/National Natural Science Foundation of China
- 82271616/National Natural Science Foundation of China
- 82271616/National Natural Science Foundation of China
- 82271616/National Natural Science Foundation of China
- 82271616/National Natural Science Foundation of China
- 82271616/National Natural Science Foundation of China
- 202103000052/Guangzhou Municipal Science and Technology Program key projects
- 202103000052/Guangzhou Municipal Science and Technology Program key projects
- 202103000052/Guangzhou Municipal Science and Technology Program key projects
- 202103000052/Guangzhou Municipal Science and Technology Program key projects
- 202103000052/Guangzhou Municipal Science and Technology Program key projects
LinkOut - more resources
Full Text Sources
Research Materials

