SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147
- PMID: 38965547
- PMCID: PMC11223399
- DOI: 10.1186/s12964-024-01718-3
SARS-CoV-2-associated lymphopenia: possible mechanisms and the role of CD147
Abstract
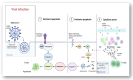
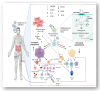
T lymphocytes play a primary role in the adaptive antiviral immunity. Both lymphocytosis and lymphopenia were found to be associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While lymphocytosis indicates an active anti-viral response, lymphopenia is a sign of poor prognosis. T-cells, in essence, rarely express ACE2 receptors, making the cause of cell depletion enigmatic. Moreover, emerging strains posed an immunological challenge, potentially alarming for the next pandemic. Herein, we review how possible indirect and direct key mechanisms could contribute to SARS-CoV-2-associated-lymphopenia. The fundamental mechanism is the inflammatory cytokine storm elicited by viral infection, which alters the host cell metabolism into a more acidic state. This "hyperlactic acidemia" together with the cytokine storm suppresses T-cell proliferation and triggers intrinsic/extrinsic apoptosis. SARS-CoV-2 infection also results in a shift from steady-state hematopoiesis to stress hematopoiesis. Even with low ACE2 expression, the presence of cholesterol-rich lipid rafts on activated T-cells may enhance viral entry and syncytia formation. Finally, direct viral infection of lymphocytes may indicate the participation of other receptors or auxiliary proteins on T-cells, that can work alone or in concert with other mechanisms. Therefore, we address the role of CD147-a novel route-for SARS-CoV-2 and its new variants. CD147 is not only expressed on T-cells, but it also interacts with other co-partners to orchestrate various biological processes. Given these features, CD147 is an appealing candidate for viral pathogenicity. Understanding the molecular and cellular mechanisms behind SARS-CoV-2-associated-lymphopenia will aid in the discovery of potential therapeutic targets to improve the resilience of our immune system against this rapidly evolving virus.
Keywords: CD147 receptor; COVID-19; Lymphopenia; T-cells SARS-CoV-2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Soybean Trypsin Inhibitor Possesses Potency Against SARS-CoV-2 Infection by Blocking the Host Cell Surface Receptors ACE2, TMPRSS2, and CD147.Int J Mol Sci. 2025 Jul 9;26(14):6583. doi: 10.3390/ijms26146583. Int J Mol Sci. 2025. PMID: 40724833 Free PMC article.
-
Determinants of susceptibility to SARS-CoV-2 infection in murine ACE2.J Virol. 2025 Jun 17;99(6):e0054325. doi: 10.1128/jvi.00543-25. Epub 2025 May 12. J Virol. 2025. PMID: 40353671 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Quantitative characterisation of extracellular vesicles designed to decoy or compete with SARS-CoV-2 reveals differential mode of action across variants of concern and highlights the diversity of Omicron.Cell Commun Signal. 2025 Jul 2;23(1):323. doi: 10.1186/s12964-025-02223-x. Cell Commun Signal. 2025. PMID: 40604989 Free PMC article.
Cited by
-
COVID-19 induces persistent transcriptional changes in adipose tissue that are not associated with Long COVID.bioRxiv [Preprint]. 2025 May 27:2025.05.23.655815. doi: 10.1101/2025.05.23.655815. bioRxiv. 2025. PMID: 40501971 Free PMC article. Preprint.
-
Soybean Trypsin Inhibitor Possesses Potency Against SARS-CoV-2 Infection by Blocking the Host Cell Surface Receptors ACE2, TMPRSS2, and CD147.Int J Mol Sci. 2025 Jul 9;26(14):6583. doi: 10.3390/ijms26146583. Int J Mol Sci. 2025. PMID: 40724833 Free PMC article.
-
Association between advanced lung cancer inflammation index and gallstone prevalence among U.S. adults: A population-based study.PLoS One. 2025 Apr 15;20(4):e0321733. doi: 10.1371/journal.pone.0321733. eCollection 2025. PLoS One. 2025. PMID: 40233085 Free PMC article.
-
Hematologic and Immunologic Overlap Between COVID-19 and Idiopathic Pulmonary Fibrosis.J Clin Med. 2025 Jul 24;14(15):5229. doi: 10.3390/jcm14155229. J Clin Med. 2025. PMID: 40806851 Free PMC article. Review.
-
EFR3A, an Intriguing Gene, and Protein with a Scaffolding Function.Cells. 2025 Mar 17;14(6):445. doi: 10.3390/cells14060445. Cells. 2025. PMID: 40136694 Free PMC article. Review.
References
-
- Markov PV et al. The evolution of SARS-CoV-2. 2023. 21(6): p. 361–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

