Cardiopulmonary and metabolic responses during a 2-day CPET in myalgic encephalomyelitis/chronic fatigue syndrome: translating reduced oxygen consumption to impairment status to treatment considerations
- PMID: 38965566
- PMCID: PMC11229500
- DOI: 10.1186/s12967-024-05410-5
Cardiopulmonary and metabolic responses during a 2-day CPET in myalgic encephalomyelitis/chronic fatigue syndrome: translating reduced oxygen consumption to impairment status to treatment considerations
Abstract
Background: Post-exertional malaise (PEM), the hallmark symptom of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), represents a constellation of abnormal responses to physical, cognitive, and/or emotional exertion including profound fatigue, cognitive dysfunction, and exertion intolerance, among numerous other maladies. Two sequential cardiopulmonary exercise tests (2-d CPET) provide objective evidence of abnormal responses to exertion in ME/CFS but validated only in studies with small sample sizes. Further, translation of results to impairment status and approaches to symptom reduction are lacking.
Methods: Participants with ME/CFS (Canadian Criteria; n = 84) and sedentary controls (CTL; n = 71) completed two CPETs on a cycle ergometer separated by 24 h. Two-way repeated measures ANOVA compared CPET measures at rest, ventilatory/anaerobic threshold (VAT), and peak effort between phenotypes and CPETs. Intraclass correlations described stability of CPET measures across tests, and relevant objective CPET data indicated impairment status. A subset of case-control pairs (n = 55) matched for aerobic capacity, age, and sex, were also analyzed.
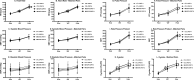
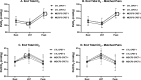
Results: Unlike CTL, ME/CFS failed to reproduce CPET-1 measures during CPET-2 with significant declines at peak exertion in work, exercise time, e, O2, CO2, T, HR, O2pulse, DBP, and RPP. Likewise, CPET-2 declines were observed at VAT for e/ CO2, PetCO2, O2pulse, work, O2 and SBP. Perception of effort (RPE) exceeded maximum effort criteria for ME/CFS and CTL on both CPETs. Results were similar in matched pairs. Intraclass correlations revealed greater stability in CPET variables across test days in CTL compared to ME/CFS owing to CPET-2 declines in ME/CFS. Lastly, CPET-2 data signaled more severe impairment status for ME/CFS compared to CPET-1.
Conclusions: Presently, this is the largest 2-d CPET study of ME/CFS to substantiate impaired recovery in ME/CFS following an exertional stressor. Abnormal post-exertional CPET responses persisted compared to CTL matched for aerobic capacity, indicating that fitness level does not predispose to exertion intolerance in ME/CFS. Moreover, contributions to exertion intolerance in ME/CFS by disrupted cardiac, pulmonary, and metabolic factors implicates autonomic nervous system dysregulation of blood flow and oxygen delivery for energy metabolism. The observable declines in post-exertional energy metabolism translate notably to a worsening of impairment status. Treatment considerations to address tangible reductions in physiological function are proffered.
Trial registration number: ClinicalTrials.gov, retrospectively registered, ID# NCT04026425, date of registration: 2019-07-17.
Keywords: Autonomic dysfunction; Cardiopulmonary exercise test; Chronic fatigue syndrome; Fatigue; Functional impairment; ME/CFS; Post exertional malaise; Two-day CPET.
© 2024. The Author(s).
Conflict of interest statement
BK, CR, JS, and SS conduct 2-day cardiopulmonary exercise testing on a fee for service basis.
Figures



Similar articles
-
Recovery from Exercise in Persons with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).Medicina (Kaunas). 2023 Mar 15;59(3):571. doi: 10.3390/medicina59030571. Medicina (Kaunas). 2023. PMID: 36984572 Free PMC article.
-
Cardiopulmonary Exercise Test Methodology for Assessing Exertion Intolerance in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.Front Pediatr. 2018 Sep 4;6:242. doi: 10.3389/fped.2018.00242. eCollection 2018. Front Pediatr. 2018. PMID: 30234078 Free PMC article.
-
Inability of myalgic encephalomyelitis/chronic fatigue syndrome patients to reproduce VO₂peak indicates functional impairment.J Transl Med. 2014 Apr 23;12:104. doi: 10.1186/1479-5876-12-104. J Transl Med. 2014. PMID: 24755065 Free PMC article.
-
Chronotropic Intolerance: An Overlooked Determinant of Symptoms and Activity Limitation in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome?Front Pediatr. 2019 Mar 22;7:82. doi: 10.3389/fped.2019.00082. eCollection 2019. Front Pediatr. 2019. PMID: 30968005 Free PMC article. Review.
-
The Prospects of the Two-Day Cardiopulmonary Exercise Test (CPET) in ME/CFS Patients: A Meta-Analysis.J Clin Med. 2020 Dec 14;9(12):4040. doi: 10.3390/jcm9124040. J Clin Med. 2020. PMID: 33327624 Free PMC article. Review.
Cited by
-
Rehabilitation management of the chronic pain-hypertension synergy: Proposal of an evidence-informed framework.Braz J Phys Ther. 2025 Jun 3;29(5):101229. doi: 10.1016/j.bjpt.2025.101229. Online ahead of print. Braz J Phys Ther. 2025. PMID: 40466275 Free PMC article. Review.
-
Exertional Exhaustion (Post-Exertional Malaise, PEM) Evaluated by the Effects of Exercise on Cerebrospinal Fluid Metabolomics-Lipidomics and Serine Pathway in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.Int J Mol Sci. 2025 Feb 1;26(3):1282. doi: 10.3390/ijms26031282. Int J Mol Sci. 2025. PMID: 39941050 Free PMC article.
-
The impact of long COVID on physical and cardiorespiratory parameters: A systematic review.PLoS One. 2025 Jun 4;20(6):e0318707. doi: 10.1371/journal.pone.0318707. eCollection 2025. PLoS One. 2025. PMID: 40465774 Free PMC article.
-
Extracellular vesicle proteomics uncovers energy metabolism, complement system, and endoplasmic reticulum stress response dysregulation postexercise in males with myalgic encephalomyelitis/chronic fatigue syndrome.Clin Transl Med. 2025 May;15(5):e70346. doi: 10.1002/ctm2.70346. Clin Transl Med. 2025. PMID: 40465195 Free PMC article.
-
Physical Activity and Sedentary Behaviour in People with Long COVID: A Follow-Up from 12 to 18 Months After Discharge.J Clin Med. 2025 May 22;14(11):3641. doi: 10.3390/jcm14113641. J Clin Med. 2025. PMID: 40507399 Free PMC article.
References
-
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/ChronicFatigue Syndrome, Board on the Health of Select Populations, Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness [Internet]. Washington (DC): National Academies Press (US). 2015. http://www.ncbi.nlm.nih.gov/books/NBK274235/. Accessed 28 Aug 2023.
-
- Davenport TE, Stevens SR, Stevens J, Snell CR, Van Ness JM. Properties of measurements obtained during cardiopulmonary exercise testing in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Work Read Mass. 2020;66:247–256. - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

