How intervention studies measure the effectiveness of medication safety-related clinical decision support systems in primary and long-term care: a systematic review
- PMID: 38965569
- PMCID: PMC11225126
- DOI: 10.1186/s12911-024-02596-y
How intervention studies measure the effectiveness of medication safety-related clinical decision support systems in primary and long-term care: a systematic review
Abstract
Background: Medication errors and associated adverse drug events (ADE) are a major cause of morbidity and mortality worldwide. In recent years, the prevention of medication errors has become a high priority in healthcare systems. In order to improve medication safety, computerized Clinical Decision Support Systems (CDSS) are increasingly being integrated into the medication process. Accordingly, a growing number of studies have investigated the medication safety-related effectiveness of CDSS. However, the outcome measures used are heterogeneous, leading to unclear evidence. The primary aim of this study is to summarize and categorize the outcomes used in interventional studies evaluating the effects of CDSS on medication safety in primary and long-term care.
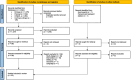
Methods: We systematically searched PubMed, Embase, CINAHL, and Cochrane Library for interventional studies evaluating the effects of CDSS targeting medication safety and patient-related outcomes. We extracted methodological characteristics, outcomes and empirical findings from the included studies. Outcomes were assigned to three main categories: process-related, harm-related, and cost-related. Risk of bias was assessed using the Evidence Project risk of bias tool.
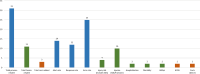
Results: Thirty-two studies met the inclusion criteria. Almost all studies (n = 31) used process-related outcomes, followed by harm-related outcomes (n = 11). Only three studies used cost-related outcomes. Most studies used outcomes from only one category and no study used outcomes from all three categories. The definition and operationalization of outcomes varied widely between the included studies, even within outcome categories. Overall, evidence on CDSS effectiveness was mixed. A significant intervention effect was demonstrated by nine of fifteen studies with process-related primary outcomes (60%) but only one out of five studies with harm-related primary outcomes (20%). The included studies faced a number of methodological problems that limit the comparability and generalizability of their results.
Conclusions: Evidence on the effectiveness of CDSS is currently inconclusive due in part to inconsistent outcome definitions and methodological problems in the literature. Additional high-quality studies are therefore needed to provide a comprehensive account of CDSS effectiveness. These studies should follow established methodological guidelines and recommendations and use a comprehensive set of harm-, process- and cost-related outcomes with agreed-upon and consistent definitions.
Prospero registration: CRD42023464746.
Keywords: Decision support systems, clinical; Medical Order Entry systems; Medication errors; Outcome Assessment (Health Care); Primary Health Care; Systematic review.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Assiri GA, Shebl NA, Mahmoud MA, Aloudah N, Grant E, Aljadhey H, Sheikh A. What is the epidemiology of medication errors, error-related adverse events and risk factors for errors in adults managed in community care contexts? A systematic review of the international literature. BMJ Open. 2018;8:e019101. doi: 10.1136/bmjopen-2017-019101. - DOI - PMC - PubMed
-
- Payne R, Slight S, Franklin BD, Avery AJ. Medication errors. Geneva: World Health Organization; 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous