Increased secretion of adipocyte-derived extracellular vesicles is associated with adipose tissue inflammation and the mobilization of excess lipid in human obesity
- PMID: 38965596
- PMCID: PMC11225216
- DOI: 10.1186/s12967-024-05249-w
Increased secretion of adipocyte-derived extracellular vesicles is associated with adipose tissue inflammation and the mobilization of excess lipid in human obesity
Abstract
Background: Obesity is a worldwide epidemic characterized by adipose tissue (AT) inflammation. AT is also a source of extracellular vesicles (EVs) that have recently been implicated in disorders related to metabolic syndrome. However, our understanding of mechanistic aspect of obesity's impact on EV secretion from human AT remains limited.
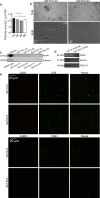
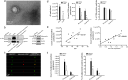
Methods: We investigated EVs from human Simpson Golabi Behmel Syndrome (SGBS) adipocytes, and from AT as well as plasma of subjects undergoing bariatric surgery. SGBS cells were treated with TNFα, palmitic acid, and eicosapentaenoic acid. Various analyses, including nanoparticle tracking analysis, electron microscopy, high-resolution confocal microscopy, and gas chromatography-mass spectrometry, were utilized to study EVs. Plasma EVs were analyzed with imaging flow cytometry.
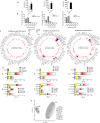
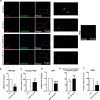
Results: EVs from mature SGBS cells differed significantly in size and quantity compared to preadipocytes, disagreeing with previous findings in mouse adipocytes and indicating that adipogenesis promotes EV secretion in human adipocytes. Inflammatory stimuli also induced EV secretion, and altered EV fatty acid (FA) profiles more than those of cells, suggesting the role of EVs as rapid responders to metabolic shifts. Visceral AT (VAT) exhibited higher EV secretion compared to subcutaneous AT (SAT), with VAT EV counts positively correlating with plasma triacylglycerol (TAG) levels. Notably, the plasma EVs of subjects with obesity contained a higher number of adiponectin-positive EVs than those of lean subjects, further demonstrating higher AT EV secretion in obesity. Moreover, plasma EV counts of people with obesity positively correlated with body mass index and TNF expression in SAT, connecting increased EV secretion with AT expansion and inflammation. Finally, EVs from SGBS adipocytes and AT contained TAGs, and EV secretion increased despite signs of less active lipolytic pathways, indicating that AT EVs could be involved in the mobilization of excess lipids into circulation.
Conclusions: We are the first to provide detailed FA profiles of human AT EVs. We report that AT EV secretion increases in human obesity, implicating their role in TAG transport and association with adverse metabolic parameters, thereby emphasizing their role in metabolic disorders. These findings promote our understanding of the roles that EVs play in human AT biology and metabolic disorders.
Keywords: Adipocyte; Adipose tissue; Extracellular vesicles; Fatty acids; Inflammation; Obesity.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures


References
-
- World Health Organization (WHO). Obesity and overweight, Key facts. 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
-
- Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;219:1–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

