lncRNA FGD5-AS1 is required for gastric cancer proliferation by inhibiting cell senescence and ROS production via stabilizing YBX1
- PMID: 38965605
- PMCID: PMC11225384
- DOI: 10.1186/s13046-024-03103-x
lncRNA FGD5-AS1 is required for gastric cancer proliferation by inhibiting cell senescence and ROS production via stabilizing YBX1
Abstract
Background: The vast majority of lncRNAs have low expression abundance, which greatly limits their functional range and impact. As a high expression abundance lncRNA, FGD5-AS1's non-ceRNA biological function in cancer is unclear.
Methods: RNA-seq studies and chromatin immunoprecipitation (Chip) assays were performed to identify ZEB1-regulated lncRNAs. RNA sequencing, RNA pulldown, RNA Immunoprecipitation assays, and rescue assays were conducted to explore the molecular mechanisms of FGD5-AS1 in GC.
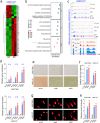
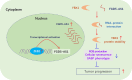
Results: As one of the most abundant lncRNAs in cells, FGD5-AS1 has been shown to be transcriptionally activated by ZEB1, thus closely related to epithelial-mesenchymal transition (EMT) signaling. Clinical analysis showed that FGD5-AS1 overexpression was clinically associated with lymph node metastasis, and predicted poor survival in GC. Loss-of-function studies confirmed that FGD5-AS1 knockdown inhibited GC proliferation and induced cisplatin chemosensibility, cell senescence, and DNA damage in GC cells. Mechanismically, FGD5-AS1 is a YBX1-binding lncRNA due to its mRNA contains three adjacent structural motifs (UAAUCCCA, ACCAGCCU, and CAGUGAGC) that can be recognized and bound by YBX1. And this RNA-protein interaction prolonged the half-life of the YBX1 protein in GC. Additionally, a rescue assay showed that FGD5-AS1 promotes GC by repressing cell senescence and ROS production via YBX1.
Conclusion: FGD5-AS1 is a cellular high-abundant lncRNA that is transcriptionally regulated by ZEB1. FGD5-AS1 overexpression promoted GC progression by inhibiting cell senescence and ROS production through binding and stabilizing the YBX1 protein.
Keywords: Cell senescence; Gastric cancer; RNA-protein interaction; ROS; Transcriptional regulation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

