Growth differentiation factor 15 is not modified after weight loss induced by liraglutide in South Asians and Europids with type 2 diabetes mellitus
- PMID: 38965822
- PMCID: PMC11291866
- DOI: 10.1113/EP091815
Growth differentiation factor 15 is not modified after weight loss induced by liraglutide in South Asians and Europids with type 2 diabetes mellitus
Abstract
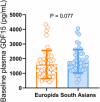
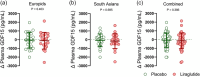
Glucagon-like peptide-1 receptor (GLP-1R) agonists induce weight loss in patients with type 2 diabetes mellitus (T2DM), but the underlying mechanism is unclear. Recently, the mechanism by which metformin induces weight loss could be explained by an increase in growth differentiation factor 15 (GDF15), which suppresses appetite. Therefore, we aimed to investigate whether the GLP-1R agonist liraglutide modifies plasma GDF15 levels in patients with T2DM. GDF15 levels were measured in plasma samples obtained from Dutch Europids and Dutch South Asians with T2DM before and after 26 weeks of treatment with daily liraglutide (n = 44) or placebo (n = 50) added to standard care. At baseline, circulating GDF15 levels did not differ between South Asians and Europids with T2DM. Treatment with liraglutide, compared to placebo, decreased body weight, but did not modify plasma GDF15 levels in all patients, or when data were split by ethnicity. Also, the change in plasma GDF15 levels after treatment with liraglutide did not correlate with changes in body weight or HbA1c levels. In addition, the dose of metformin used did not correlate with baseline plasma GDF15 levels. Compared to placebo, liraglutide treatment for 26 weeks does not modify plasma GDF15 levels in Dutch Europid or South Asian patients with T2DM. Thus, the weight loss induced by liraglutide is likely explained by other mechanisms beyond the GDF15 pathway. HIGHLIGHTS: What is the central question of this study? Growth differentiation factor 15 (GDF15) suppresses appetite and is increased by metformin: does the GLP-1R agonist liraglutide modify plasma GDF15 levels in patients with type 2 diabetes mellitus (T2DM)? What is the main finding and its importance? Plasma GDF15 levels did not differ between South Asians and Europids with T2DM and were not modified by 26 weeks of liraglutide in either ethnicity. Moreover, there was no correlation between the changes in plasma GDF15 levels and dosage of metformin administered, changes in body weight or HbA1c levels. The appetite-suppressing effect of liraglutide is likely exerted via pathways other than GDF15.
Keywords: ethnic differences; metabolic diseases; obesity; weight‐reducing drugs.
© 2024 The Author(s). Experimental Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declared no conflict of interest.
Figures




References
-
- American Diabetes Association Professional Practice Committee . (2021). 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes—2022. Diabetes Care, 45, (Supplement_1), S125–S143. - PubMed
-
- Anjana, R. M. , Pradeepa, R. , Deepa, M. , Datta, M. , Sudha, V. , Unnikrishnan, R. , Bhansali, A. , Joshi, S. R. , Joshi, P. P. , Yajnik, C. S. , Dhandhania, V. K. , Nath, L. M. , Das, A. K. , Rao, P. V. , Madhu, S. V. , Shukla, D. K. , Kaur, T. , Priya, M. , Nirmal, E. , … Mohan, V. (2011). Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research‐INdia DIABetes (ICMR‐INDIAB) study. Diabetologia, 54(12), 3022–3027. - PubMed
-
- Association, W. M. (2013). World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. Journal of the American Medical Association, 310(20), 2191–2194. - PubMed
-
- Baek, S. J. , Kim, K. S. , Nixon, J. B. , Wilson, L. C. , & Eling, T. E. (2001). Cyclooxygenase inhibitors regulate the expression of a TGF‐beta superfamily member that has proapoptotic and antitumorigenic activities. Molecular Pharmacology, 59(4), 901–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
