Modeling the Effects of Protracted Cosmic Radiation in a Human Organ-on-Chip Platform
- PMID: 38965824
- PMCID: PMC11558103
- DOI: 10.1002/advs.202401415
Modeling the Effects of Protracted Cosmic Radiation in a Human Organ-on-Chip Platform
Abstract
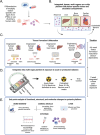
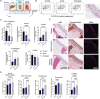
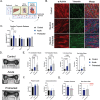
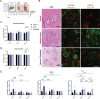
Galactic cosmic radiation (GCR) is one of the most serious risks posed to astronauts during missions to the Moon and Mars. Experimental models capable of recapitulating human physiology are critical to understanding the effects of radiation on human organs and developing radioprotective measures against space travel exposures. The effects of systemic radiation are studied using a multi-organ-on-a-chip (multi-OoC) platform containing engineered tissue models of human bone marrow (site of hematopoiesis and acute radiation damage), cardiac muscle (site of chronic radiation damage) and liver (site of metabolism), linked by vascular circulation with an endothelial barrier separating individual tissue chambers from the vascular perfusate. Following protracted neutron radiation, the most damaging radiation component in deep space, a greater deviation of tissue function is observed as compared to the same cumulative dose delivered acutely. Further, by characterizing engineered bone marrow (eBM)-derived immune cells in circulation, 58 unique genes specific to the effects of protracted neutron dosing are identified, as compared to acutely irradiated and healthy tissues. It propose that this bioengineered platform allows studies of human responses to extended radiation exposure in an "astronaut-on-a-chip" model that can inform measures for mitigating cosmic radiation injury.
Keywords: bone marrow; heart; human stem cells; liver; mars mission; organ‐on‐chip; radiation; tissue engineering.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bokhari R. S., Beheshti A., Blutt S. E., Bowles D. E., Brenner D., Britton R., Bronk L., Cao X.u, Chatterjee A., Clay D. E., Courtney C., Fox D. T., Gaber M. W., Gerecht S., Grabham P., Grosshans D., Guan F., Jezuit E. A., Kirsch D. G., Liu Z., Maletic‐Savatic M., Miller K. M., Montague R. A., Nagpal P., Osenberg S., Parkitny L., Pierce N. A., Porada C., Rosenberg S. M., Sargunas P., et al., Life Sci. Space Res. 2022, 35, 105. - PubMed
-
- Krestinina L. Y., Preston D. L., Ostroumova E. V., Degteva M. O., Ron E., Vyushkova O. V., Startsev N. V., Kossenko M. M., Akleyev A. V., Radiat. Res. 2005, 164, 602. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
