Many locks to one key: N-acetylneuraminic acid binding to proteins
- PMID: 38965900
- PMCID: PMC11364026
- DOI: 10.1107/S2052252524005360
Many locks to one key: N-acetylneuraminic acid binding to proteins
Abstract
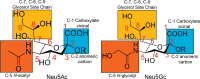
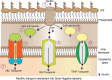
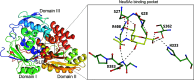
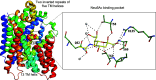
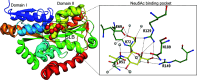
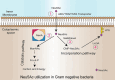
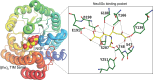
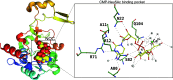
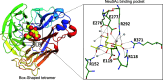
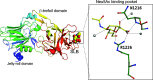
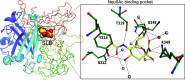
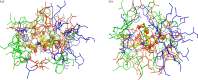
Sialic acids play crucial roles in cell surface glycans of both eukaryotic and prokaryotic organisms, mediating various biological processes, including cell-cell interactions, development, immune response, oncogenesis and host-pathogen interactions. This review focuses on the β-anomeric form of N-acetylneuraminic acid (Neu5Ac), particularly its binding affinity towards various proteins, as elucidated by solved protein structures. Specifically, we delve into the binding mechanisms of Neu5Ac to proteins involved in sequestering and transporting Neu5Ac in Gram-negative bacteria, with implications for drug design targeting these proteins as antimicrobial agents. Unlike the initial assumptions, structural analyses revealed significant variability in the Neu5Ac binding pockets among proteins, indicating diverse evolutionary origins and binding modes. By comparing these findings with existing structures from other systems, we can effectively highlight the intricate relationship between protein structure and Neu5Ac recognition, emphasizing the need for tailored drug design strategies to inhibit Neu5Ac-binding proteins across bacterial species.
Keywords: Gram-negative bacteria; N-acetyl neuraminic acid; Neu5Ac; binding sites; drug discovery; molecular recognition; protein structures; sialic acids.
open access.
Figures


Similar articles
-
Uncovering a novel molecular mechanism for scavenging sialic acids in bacteria.J Biol Chem. 2020 Oct 2;295(40):13724-13736. doi: 10.1074/jbc.RA120.014454. Epub 2020 Jul 15. J Biol Chem. 2020. PMID: 32669363 Free PMC article.
-
Exploring the Impact of Ketodeoxynonulosonic Acid in Host-Pathogen Interactions Using Uptake and Surface Display by Nontypeable Haemophilus influenzae.mBio. 2021 Jan 19;12(1):e03226-20. doi: 10.1128/mBio.03226-20. mBio. 2021. PMID: 33468699 Free PMC article.
-
Glycoengineering with neuraminic acid analogs to label lipooligosaccharides and detect native sialyltransferase activity in gram-negative bacteria.Glycobiology. 2024 Aug 30;34(10):cwae071. doi: 10.1093/glycob/cwae071. Glycobiology. 2024. PMID: 39244665 Free PMC article.
-
Sialic acid diversity in the human gut: Molecular impacts and tools for future discovery.Curr Opin Struct Biol. 2022 Aug;75:102397. doi: 10.1016/j.sbi.2022.102397. Epub 2022 May 30. Curr Opin Struct Biol. 2022. PMID: 35653953 Review.
-
How bacteria utilize sialic acid during interactions with the host: snip, snatch, dispatch, match and attach.Microbiology (Reading). 2022 Mar;168(3):001157. doi: 10.1099/mic.0.001157. Microbiology (Reading). 2022. PMID: 35316172 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

