REVIEW OF ADVERSE DRUG REACTIONS OF MEDICINES USED FOR THE TREATMENT OF BENIGN PROSTATIC HYPERPLASIA REPORTED TO HALMED
- PMID: 38966030
- PMCID: PMC11221238
- DOI: 10.20471/acc.2023.62.s2.10
REVIEW OF ADVERSE DRUG REACTIONS OF MEDICINES USED FOR THE TREATMENT OF BENIGN PROSTATIC HYPERPLASIA REPORTED TO HALMED
Abstract
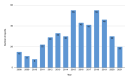
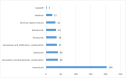
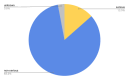
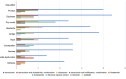
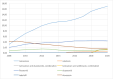
Benign prostatic hyperplasia is one of the most common diseases in men, with a prevalence rate of 50% in their 50s to 80% in their 80s, and is mostly treated with chronic drug therapy. The aim of this study was to analyze adverse drug reactions (ADR) to drugs used in benign prostate hyperplasia (BPH) treatment reported to HALMED from 2008 to 2021. Data on ADR reports in Croatia were obtained from the VigiFlow national database and on the use of drugs for BPH in Croatia from Drug Utilization Reports from HALMED. In the observed period, the number of reports on each BPH drug, total number of reports, seriousness of reported ADR, patient age and sex, type of reporter, and most reported ADRs were analyzed. Results showed that 438 ADR reports were received, of which 45.95% on tamsulosin as the most frequently used drug for BPH. Of all reports, 84% were non-serious, 96% were reported in men and 82% in patients older than 45 years. The most frequently reported ADRs were consistent with the known safety profile of BPH drugs. Pharmacists were the most common (47%) reporters of ADRs for BPH drugs, while 33% were reported by physicians. Analysis of the reported ADRs showed that most frequently reported ones were in line with the known safety profile of BPH drugs. However, given the prevalence of the disease and the extent of the use of BPH drugs, it could be argued that the number of reports could be higher (i.e., 34 reports/year). Reporting on ADRs is necessary to better understand the safety profile of drugs in the post-authorization period, and more information on the safe use of medicines could be collected by raising awareness of healthcare professionals.
Keywords: Benign prostate hyperplasia; Combination of tamsulosin and dutasteride; Combination of tamsulosin and solifenacin; Doxazosin; Dutasteride; Finasteride; Serenoa repens extract; Side effects; Silodosin; Tadalafil; Tamsulosin.
Sestre Milosrdnice University Hospital.
Figures


References
-
- Guidelines EAU. Edn. presented at the EAU Annual Congress Amsterdam 2022. ISBN 978-94-92671-16-5. Arnhem, The Netherlands: EAU Guidelines Office.
-
- EudraVigilance – European database of suspected adverse drug reaction reports. Available from: https://www.adrreports.eu/en/. (Accessed 20 Apr 2022)
-
- The use of the WHO-UMC system for standardised case causality assessment. Available from: https://who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf. (Accessed 20 Apr 2022)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
