Recent advances in the development of tumor microenvironment-activatable nanomotors for deep tumor penetration
- PMID: 38966042
- PMCID: PMC11222818
- DOI: 10.1016/j.mtbio.2024.101119
Recent advances in the development of tumor microenvironment-activatable nanomotors for deep tumor penetration
Abstract
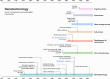
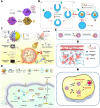
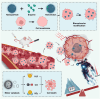
Cancer represents a significant threat to human health, with the use of traditional chemotherapy drugs being limited by their harsh side effects. Tumor-targeted nanocarriers have emerged as a promising solution to this problem, as they can deliver drugs directly to the tumor site, improving drug effectiveness and reducing adverse effects. However, the efficacy of most nanomedicines is hindered by poor penetration into solid tumors. Nanomotors, capable of converting various forms of energy into mechanical energy for self-propelled movement, offer a potential solution for enhancing drug delivery to deep tumor regions. External force-driven nanomotors, such as those powered by magnetic fields or ultrasound, provide precise control but often necessitate bulky and costly external equipment. Bio-driven nanomotors, propelled by sperm, macrophages, or bacteria, utilize biological molecules for self-propulsion and are well-suited to the physiological environment. However, they are constrained by limited lifespan, inadequate speed, and potential immune responses. To address these issues, nanomotors have been engineered to propel themselves forward by catalyzing intrinsic "fuel" in the tumor microenvironment. This mechanism facilitates their penetration through biological barriers, allowing them to reach deep tumor regions for targeted drug delivery. In this regard, this article provides a review of tumor microenvironment-activatable nanomotors (fueled by hydrogen peroxide, urea, arginine), and discusses their prospects and challenges in clinical translation, aiming to offer new insights for safe, efficient, and precise treatment in cancer therapy.
Keywords: Arginine; Deep tumor penetration; Hydrogen peroxide; Nanomotors; Self-propelled; Urea.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Multi-phoretic nanomotor with consistent motion direction for enhanced cancer therapy.Acta Biomater. 2025 Jan 1;191:352-368. doi: 10.1016/j.actbio.2024.11.037. Epub 2024 Nov 24. Acta Biomater. 2025. PMID: 39586348
-
Photothermal interference urease-powered polydopamine nanomotor for enhanced propulsion and synergistic therapy.Colloids Surf B Biointerfaces. 2022 Apr;212:112353. doi: 10.1016/j.colsurfb.2022.112353. Epub 2022 Jan 22. Colloids Surf B Biointerfaces. 2022. PMID: 35085936
-
Janus micro/nanomotors for enhanced disease treatment through their deep penetration capability.Acta Biomater. 2025 Apr;196:50-77. doi: 10.1016/j.actbio.2025.02.055. Epub 2025 Feb 25. Acta Biomater. 2025. PMID: 40015356 Review.
-
Dual-Fuel Propelled Nanomotors with Two-Stage Permeation for Deep Bacterial Infection in the Treatment of Pulpitis.Adv Sci (Weinh). 2024 Feb;11(5):e2305063. doi: 10.1002/advs.202305063. Epub 2023 Dec 3. Adv Sci (Weinh). 2024. PMID: 38044274 Free PMC article.
-
Unfolding the future: Self-controlled catalytic nanomotor in healthcare system.Mater Sci Eng C Mater Biol Appl. 2020 Dec;117:111330. doi: 10.1016/j.msec.2020.111330. Epub 2020 Aug 7. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32919683 Review.
Cited by
-
Excipients for Cerium Dioxide Nanoparticle Stabilization in the Perspective of Biomedical Applications.Molecules. 2025 Mar 8;30(6):1210. doi: 10.3390/molecules30061210. Molecules. 2025. PMID: 40141988 Free PMC article. Review.
-
FBXO42 promotes hepatocellular carcinoma progression via mediating p57Kip2 ubiquitination and degradation.Eur J Med Res. 2025 Aug 21;30(1):784. doi: 10.1186/s40001-025-03050-z. Eur J Med Res. 2025. PMID: 40842039 Free PMC article.
References
-
- Zheng R.S., et al. Cancer incidence and mortality in China, 2016. Journal of the National Cancer Center. 2022;2:1–9.
-
- Falkson C.B., et al. Surgical, radiation, and systemic treatments of patients with thymic epithelial tumors: a systematic review. J. Thorac. Oncol. 2023;18:299–312. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

