Anti-allergic effect of Cyclopia (honeybush) extracts via anti-degranulation activity in a murine allergy model for inhaled antigen
- PMID: 38966058
- PMCID: PMC11220329
- DOI: 10.12938/bmfh.2023-105
Anti-allergic effect of Cyclopia (honeybush) extracts via anti-degranulation activity in a murine allergy model for inhaled antigen
Abstract
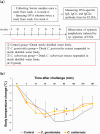
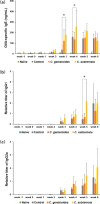
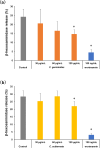
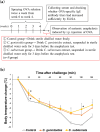
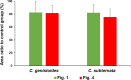
The anti-allergic effects of extracts prepared from two species of honeybush, Cyclopia genistoides and Cyclopia subternata, were demonstrated in vivo in a murine allergy model for inhaled antigen induced with ovalbumin (OVA) inhalation to mimic pollen allergy. Intake of the extracts increased the production of OVA-specific immunoglobulin (Ig) E (IgE), IgG1, and IgG2a antibodies in serum and significantly suppressed anaphylactic reaction-induced body temperature decline. Moreover, the extracts significantly inhibited antigen-antibody-induced degranulation in RBL-2H3 cells. They also inhibited body temperature decline when the allergic mice were given them after antigen sensitization, indicating that anti-degranulation activity is the major mechanism underlying the anti-allergic effect of Cyclopia extracts. Despite their qualitative and quantitative differences in phenolic composition, the two extracts exhibited similar effects, suggesting that several active compounds might be involved in the activity. Therefore, oral administration of either Cyclopia extract potentially exerts a systemic anti-allergic effect, supporting the increased consumption of honeybush tea for general wellness and improved quality of life.
Keywords: Cyclopia; IgE; RBL-2H3 cell; anti-allergic activity; degranulation; honeybush; respiratory allergy model.
©2024 BMFH Press.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Cyclopia Extracts Enhance Th1-, Th2-, and Th17-type T Cell Responses and Induce Foxp3+ Cells in Murine Cell Culture.Planta Med. 2018 Mar;84(5):311-319. doi: 10.1055/s-0043-121270. Epub 2017 Nov 2. Planta Med. 2018. PMID: 29096404
-
Autophagy-induced cell death by aqueous and polyphenol-enriched extracts of honeybush (Cyclopia spp.) in liver and colon cancer cells.Food Sci Nutr. 2024 May 15;12(8):5647-5662. doi: 10.1002/fsn3.4214. eCollection 2024 Aug. Food Sci Nutr. 2024. PMID: 39139978 Free PMC article.
-
Biocontrol of Fusarium Species Utilizing Indigenous Rooibos and Honeybush Extracts.Microbiol Spectr. 2023 Jun 15;11(3):e0274222. doi: 10.1128/spectrum.02742-22. Epub 2023 May 24. Microbiol Spectr. 2023. PMID: 37222581 Free PMC article.
-
Cyclopia maculata and Cyclopia subternata (honeybush tea) inhibits adipogenesis in 3T3-L1 pre-adipocytes.Phytomedicine. 2013 Mar 15;20(5):401-8. doi: 10.1016/j.phymed.2012.12.002. Epub 2013 Feb 18. Phytomedicine. 2013. PMID: 23428403
-
Honeybush (Cyclopia sp.) - a rich source of compounds with high antimutagenic properties.Fitoterapia. 2009 Jan;80(1):3-11. doi: 10.1016/j.fitote.2008.11.001. Epub 2008 Nov 11. Fitoterapia. 2009. PMID: 19032980 Review.
References
-
- Bauchau V, Durham SR. 2004. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 24: 758–764. - PubMed
-
- Morais S, Tortajada-Genaro LA, Maquieira Á, Martinez MAG. 2020. Biosensors for food allergy detection according to specific IgE levels in serum. TrAC Trends Analyt Chem 127: 115904.
-
- Osada T, Okano M. 2021. Japanese cedar and cypress pollinosis updated: new allergens, cross-reactivity, and treatment. Allergol Int 70: 281–290. - PubMed
-
- Bessa C, Francisco T, Dias R, Mateus N, de Freitas V, Pérez-Gregorio R. 2021. Use of polyphenols as modulators of food allergies. From chemistry to biological implications. Front Sustain Food Syst 5: 623611.

